A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Accessing the Cytotoxicity and Cell Response to Biomaterials
* These authors contributed equally
In This Article
Summary
This methodology aims to evaluate biomaterial cytotoxicity through the preparation of soluble extracts, using viability assays and phenotypic analysis, including flow cytometry, RT-PCR, immunocytochemistry, and other cellular and molecular biology techniques.
Abstract
Biomaterials contact directly or indirectly with the human tissues, making it important to evaluate its cytotoxicity. This evaluation can be performed by several methods, but a high discrepancy exists between the approaches used, compromising the reproducibility and the comparison among the obtained results. In this paper, we propose a protocol to evaluate biomaterials cytotoxicity using soluble extracts, which we use for dental biomaterials. The extracts preparation is detailed, from pellets production to its extraction in a culture medium. The biomaterials cytotoxicity evaluation is based on metabolic activity using the MTT assay, cell viability using the Sulphorhodamine B (SBR) assay, cell death profile by flow cytometry, and cell morphology using May-Grünwald Giemsa. Additional to cytotoxicity evaluation, a protocol to evaluate cell function is described based on the expression of specific markers assessed by immunocytochemistry and PCR. This protocol provides a comprehensive guide for biomaterials cytotoxicity and cellular effects evaluation, using the extracts methodology, in a reproducible and robust manner.
Introduction
Biocompatibility can be defined as the capacity of a material to integrate tissue and induce a favorable therapeutic response, free of local and systemic damages1,2,3. Biocompatibility evaluation is crucial for the development of any material intended for medical use. Therefore, this protocol provides a systematic and comprehensive approach for every researcher aiming to develop new biomaterials or studying new applications for existing biomaterials.
In vitro cytotoxicity tests are widely used as the first phase for biocompatibility evaluation, using primary cell cultures or cell lines. The results constitute a first indicator of potential clinical application. Besides being vital for the biomaterial development, this testing is mandatory to comply with current regulations for market introduction, from EUA and EU regulators (FDA and CE certification)4,5,6,7,8. Moreover, standardized testing in biomedical research provides a significant advantage in terms of reproducibility and comparison of results from different studies on similar biomaterials or devices9.
International Organization for Standardization (ISO) guidelines are widely used by multiple independent commercial, regulatory, and academic laboratories for testing materials in an accurate and reproducible manner. The ISO 10993-5 refers to the in vitro cytotoxicity assessment and the ISO 10993-12 reports to sampling preparation10,11. For biomaterial testing three categories are provided, to be selected according to the material type, contacting tissues, and the treatment goal: extracts, direct contact, and indirect contact8,11,12,13. Extracts are obtained by enriching a cell culture medium with the biomaterial. For the direct contact tests, the biomaterial is placed directly on the cell cultures, and, in indirect contact, incubation with the cells is performed separated by a barrier, such as an agarose gel11. Appropriate controls are mandatory, and a minimum of three independent experiments should be performed5,8,10,11,14.
It is critical to simulate or exaggerate clinical conditions to determine the cytotoxic potential. In the case of extracts testing, the material's surface area; the medium volume; the medium and the material pH; the material solubility, osmolarity and diffusion ratio; and the extraction conditions such as agitation, temperature, and time influence media enrichmen5.
The methodology allows the quantitative and qualitative evaluation of cytotoxicity of several pharmaceutical formulations, both solid and liquid. Several assays can be performed, such as neutral red uptake test, colony formation test, MTT assay, and XTT assay5,10,14.
Most cytotoxicity assessment studies published use simpler assays, namely MTT and XTT, which provide limited information. Evaluating biocompatibility should not only involve the assessment of cytotoxicity but also bioactivity of a given test material2, as this protocol endorses. Additional evaluation criteria should be used when justified and documented. Thus, this protocol aims to provide a comprehensive guide, detailing a set of methods for the biomaterial cytotoxicity evaluation. Besides, the evaluation of different cellular processes, namely the type of cell death, cell morphology, cell function in the synthesis of specific proteins, and specific tissue production, are described.
Protocol
1. Pellets preparation
- Prepare the polyvinyl chloride (PVC) molds by performing circular-shaped holes of known dimensions in PVC plates.
NOTE: PVC moldings can be made of different sizes. Calculate the contact surface of PVC molds, using the formula A= h(2πr)+2πr2 (r: radius of the cylinder; h: height of the cylinder). - Prepare the biomaterial to be tested according to the manufacturer's instructions and as close as possible to the beginning of the experiment.
NOTE: For the preparation of paste/paste formulation biomaterials, an adequate amount of base paste and catalyst are mixed manually with a mixing spatula. For other materials based on liquid and powder formulations, manual spatulation or mechanical mixing with vibration should be performed, following the manufacturer's instructions or the adequate for new materials. For liquid materials, this step is not necessary. Start the protocol in step 2. - Place the biomaterial on the molds with a spatula and let them set for the appropriate time.
NOTE: The setting time and setting conditions of the biomaterials must follow the manufacturer's instructions or the adequate for new materials. - After setting, remove the biomaterial’ pellets from the PVC molds and place them in a container (a 6 well plate or a Petri dish can be used).
- Sterilize the pellets by placing them under an ultraviolet light (UV) lamp for 20 minutes for each side.
2. Obtaining the biomaterials' extracts
NOTE: All procedures should be performed under strict sterile conditions.
- Determine the necessary number of pellets by calculating the pellet surface area based on the formula described in 1.1.
NOTE: As a reference value, the contact surface area of 250 mm2/mL11,15 is achieved by adding 9 pellets (r 3 mm x h 1.5 mm) per mL of the medium. - Prepare the soluble extracts (extract enriched with the biomaterial).
- Place the pellets in a 50 mL tube and add the corresponding of the cell culture medium. Place the tubes for 24 hours in the incubator at 37°, in constant rotation.
NOTE: Use the cell culture medium appropriate for the cell cultures. - After 24 hours, remove the tubes from the incubator. At this point, the extracts correspond to a concentration of 1/1 or 100%.
- Make dilutions of the extract by sequential addition of equal volumes of conditioned medium to cell culture medium.
NOTE: No pH adjustment should be made to the media.- Add 1 mL of culture media to 1 mL of 100% extract to obtain a 50% extract. Add 1 mL of culture media to 1 mL of 50% extract to obtain a 25% extract, and so on (Figure 1).
NOTE: Use the concentrations found relevant for each compound.
- Add 1 mL of culture media to 1 mL of 100% extract to obtain a 50% extract. Add 1 mL of culture media to 1 mL of 50% extract to obtain a 25% extract, and so on (Figure 1).
- Place the pellets in a 50 mL tube and add the corresponding of the cell culture medium. Place the tubes for 24 hours in the incubator at 37°, in constant rotation.
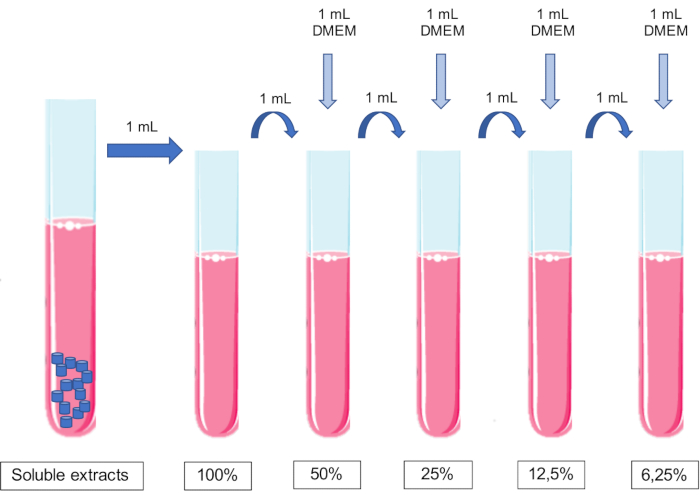
Figure 1: Scheme of the preparation and dilutions of soluble extracts. Please click here to view a larger version of this figure.
3. Cell incubation with the biomaterials’ extracts
- Prepare a cellular suspension and plate it in an adequate cell container, such as a multiwell plate, according to the number of cells needed for the experiments.
- Start with a flask of the desired cells with 80% to 90% confluence.
- Discard the cell culture media, wash with phosphate-buffered saline solution (PBS) and detach the cells with trypsin-EDTA (1 to 2 mL for a 75 cm2 cell culture flask).
- Add the cell culture media (2 to 4 mL for a 75 cm2 cell culture flask), transfer the cell suspension to a tube and centrifuge at 200 x g for 5 min.
- Suspend the pellet in a known volume of cell culture media.
NOTE: This protocol is designed for the use of adherent cell cultures; however, simple adaptations can be made to work with suspension cell cultures. - Count the cells in the hemocytometer and calculate the cell concentration of the cell suspension.
- Suspend the determined amount of cell suspension in culture medium and transfer to multiwell dishes. As a reference value for seeding density, consider 5 – 20 x 105 cells/cm2.
NOTE: The appropriated number of cells must be calculated according to the cell type and cell characteristics, namely cell doubling time.
- Incubate the cells for 24 hours to allow cell adhesion.
- After this period, administer the soluble extracts into the culture plates.
- Aspirate the cell culture medium.
- Add the biomaterials’ extracts to each well, according to the sequence of concentrations, as described previously. Add fresh cell culture medium to the control wells.
- Incubate the plates for 24 h or longer.
NOTE: Negative controls must be performed in each assay, corresponding to untreated cells, maintained in the culture medium. The incubation times can be selected accordingly to the study goals.
4. Evaluation of the metabolic activity
- After the cell incubation with the biomaterials’ extracts, aspirate the medium from the plates and wash each well PBS.
- Place, in each well, the adequate volume of 0.5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazole bromide (MTT) prepared in PBS, pH 7.4.
- Incubate the plates for 4 h or overnight in the dark at 37 ° C.
- To solubilize the obtained formazan crystals, add the adequate volume of 0.04 M solution of hydrochloric acid in isopropanol to each well and stir plates for 30 minutes.
NOTE: Adjust the amount of MTT and isopropanol according to the size of the wells. - Stir and homogenize the contents of each well, if necessary, by pipetting up and down until no crystals are seen.
- Quantify the absorbance at a wavelength of 570 nm with a 620 nm reference filter, in the spectrophotometer.
- To calculate the metabolic activity, divide the absorbance of the treated cells by the absorbance of the control cultures. To obtain percentage values multiply by 100.
5. Cell death evaluation
NOTE: To perform this evaluation a minimum of 106 cells per condition should be used.
- Use centrifuge tubes properly identified, accordingly to the conditions being evaluated.
- After the cell incubation with the biomaterials’ extracts, collect the culture media to the respective tube.
- Detach the cells and add the cell suspension to the respective tubes.
- Concentrate the cell suspensions by centrifugation at 120 x g for 5 minutes.
- Wash the pellets with PBS. Remove the PBS by centrifugation at 1,000 x g for 5 minutes.
- Add 1 mL of PBS and transfer the cell pellets to identified cytometry tubes.
- Remove the PBS by centrifugation at 1,000 x g for 5 minutes.
- Incubate with 100 µL of binding buffer (0.01 M HEPES, 0.14 mM NaCl and 0.25 mM CaCl2)16, and allow the cells to rest for about 15 minutes for cell membrane recovery.
- Add 2.5 μL of fluorescent labelled Annexin-V and 1 μL of propidium iodide for 15 minutes at room temperature in the dark.
- After incubation, add 400 μL of PBS and analyze on the cytometer. For the analysis and quantification of the information use appropriate software.
- Present results as a percentage of live cells, apoptosis, late apoptosis/necrosis, and necrosis.
6. Morphology evaluation
- Select the appropriate size of sterilized glass coverslips that fit inside the multiwell plate.
- Place each slide in a well using sterile tweezers.
- Distribute a cellular suspension at an adequate concentration into the wells and let overnight in an incubator at 37 °C in a humidified atmosphere with 95% air and 5% CO2.
- Expose the cell cultures to the extracts, as previously described.
- Aspirate the media and wash with PBS.
- Let the coverslips dry at room temperature and then add a sufficient volume of May-Grünwald solution to cover the coverslips; incubate for 3 minutes.
- Remove the dye and wash with distilled water for 1 minute.
- Remove the water and add a sufficient volume of Giemsa solution to cover the coverslips; incubate for 15 minutes.
- Wash the coverslips in running water.
- Transfer the coverslips to a slide.
- Look under a microscope. Take the photographs with the chosen magnification.
7. Cell function assessment through reverse transcription polymerase chain reaction (RT-PCR)
NOTE: To perform this evaluation a minimum of 2x106 cells per condition should be used. As an example, alkaline phosphatase is presented as a gene of interest for odontoblasts activity evaluation. Other genes of interest can be seen in Table 1.
- Plate the cells as described above.
NOTE: The concentration of cells plated might need to be adjusted, accordingly to the cell type and cytotoxicity of the biomaterials being studied. - Incubate with soluble extracts, as described above.
- Detach the cells to obtain a suspension as described before.
- Wash the cells twice with PBS; for this centrifuge at 200 x g for 5 minutes at room temperature.
- Lyse the cells by suspending the pellet in 1 mL of RNA purification solution (e.g., NZYol), intense stirring, and successive pipetting.
- Incubate the samples for 5 minutes at room temperature.
- Add 200 µL of chloroform and shake the tubes by hand for 15 seconds.
- Incubate for 3 minutes at room temperature.
- Centrifuge lysates at 4 ° C for 15 min at 12,000 x g. During this centrifugation, two phases originate in the sample, leaving the RNA in the aqueous (upper) phase.
- Remove the aqueous phase to a new tube and add 500 µL of cold isopropanol to precipitate RNA.
- Incubate samples at room temperature for 10 minutes and centrifuge at 12,000 x g for 10 minutes at 4 ° C.
- Remove the supernatant and wash the pellet with 1 mL of 75% ethanol by centrifugation at 7,500 x g for 5 minutes at 4 ° C.
- Dry the pellet at room temperature until ethanol evaporation.
- Suspend in RNase-free water.
- Quantify and determine the degree of purity of the samples using absorption spectrophotometry, at the wavelengths 260 nm and 280 nm. Determine the RNA purity and use samples with a purity ratio (A260/280) around 2.0.
- Store samples at -80 ° C.
- Proceed to perform RT-PCR following manufacturer's protocol17.
NOTE: Accordingly to the study goal, select the specific markers to be evaluated.
8. Cell function assessment through protein identification
NOTE: According to the study goal, select the specific proteins to be evaluated. As an example, dentin sialoprotein (DSP) is presented as a protein of interest for odontoblasts activity evaluation. Other proteins of interest can be seen in Table 1.
- Culture cells in coverslips and expose to the extracts, as described before.
- Wash the cell cultures with PBS.
- Fix with 3.7% paraformaldehyde for 30 minutes at room temperature.
- Wash twice with PBS.
- Permeabilize with 0.5% Triton in PBS for 15 minutes.
- Block the peroxidase with 0.3% hydrogen peroxide in PBS for 5 minutes.
- Wash twice with PBS.
- Wash twice with 0.5% bovine serum albumin (BSA).
- Block cell cultures with 2% BSA for 45 minutes.
- Wash with 0.5% BSA in PBS.
- Incubate cultures with the primary antibody according to the selected protein for 60 minutes at room temperature.
NOTE: This protocol uses the primary antibody DSP(M20) Antibody (1:100) and the secondary antibody Polyclonal Rabbit Anti-goat immunoglobulins/HRP (1:100). - Wash five times with 0.5% BSA in PBS.
- Incubate with secondary antibody for 90 minutes at room temperature.
NOTE: Make the antibody dilutions using 0.5% BSA in PBS. - Wash five times with 0.5% BSA in PBS for 1 minute in each wash.
- Incubate cultures with a substrate and chromogen mixture at a concentration of 20 µL chromogen/mL substrate for 25 minutes.
- Wash twice with 0.5% BSA in PBS.
- Counterstain with Hematoxylin for 15 minutes.
- Wash with a sequence of 0.037 mol/L ammonia and distilled water for 5 minutes to remove excess dye.
- Mount the coverslips on the slides. Use glycerol as the mounting medium.
- Allow drying overnight.
- Look under a microscope. Take the photographs with the chosen magnification.
9. Mineralization assessment through Alizarin Red S assay
- Prepare an Alizarin Red S solution at a concentration of 40 mM18. Stir the solution for homogenization for 12 hours in the dark.
NOTE: To prepare 100 mL of Alizarin Red S solution, solubilize 1.44 g of alizarin powder (Molecular weight: 360 g/mol) in ultrapure water, protected from light. For this solution, the pH value is critical and should be between 4.1 and 4.3. - Incubate cell culture with soluble extracts, as described above.
- Wash cell cultures three times with PBS.
- Fix with 4% paraformaldehyde for 15 minutes at room temperature.
- Wash three times with PBS.
- Stain with Alizarin Red Staining solution for 20 minutes at 37 °C in the dark.
- After staining, wash the plates with PBS to remove the excess dye.
- Look under a microscope. Take the photographs with the chosen magnification.
- Add an extraction solution, composed by 10% (w/v) acetic acid and 20% (w/v) methanol, to each well, and let stirring for 40 minutes at room temperature.
- Measure the absorbance at 490 nm wavelength on a spectrophotometer19.
Results
The representative results here refer to the study of dental biomaterials. The extract methodology allows to obtain a cytotoxicity profile and cell function after exposition to the dental materials, regarding effects on metabolic activity (Figure 2), cell viability, cell death profile and cell morphology (Figure 3), and specific proteins expression (Figure 4).
The MTT assay is used to obtain a quick overv...
Discussion
This protocol was designed taking into consideration the ISO 10993-5, which refers to the evaluation of in vitro cytotoxicity of biomaterials that contact with the tissues, to evaluate the biocompatibility and to contribute to studies reproducibility21. This is a growing concern in science, and many authors are already following these recommendations in the experimental design of their in vitro studies15,22,
Disclosures
The authors have no competing financial interests or other conflicts of interest.
Acknowledgements
We thank the following for support: GAI 2013 (Faculdade de Medicina da Universidade de Coimbra); CIBB is funded by National Funds via FCT (Foundation for Science and Technology) through the Strategic Project UIDB/04539/2020 and UIDP/04539/2020 (CIBB). We thank to Jacques Nör, University of Michigan Dental School, for providing the cell line MDPC-23.
Materials
| Name | Company | Catalog Number | Comments |
| Absolute ethanol | Merck Millipore | 100983 | |
| Accutase | Gibco | A1110501 | StemPro Accutas Cell Dissociation Reagent |
| ALDH antibody | Santa Cruz Biotechnology | SC166362 | |
| Annexin V FITC | BD Biosciences | 556547 | |
| Antibiotic antimycotic solution | Sigma | A5955 | |
| BCA assay | Thermo Scientific | 23225 | Pierce BCA Protein Assay Kit |
| Bovine serum albumin | Sigma | A9418 | |
| CaCl2 | Sigma | 10035-04-8 | |
| CD133 antibody | Miteny Biotec | 293C3-APC | Allophycocyanin (APC) |
| CD24 antibody | BD Biosciences | 658331 | Allophycocyanin-H7 (APC-H7) |
| CD44 antibody | Biolegend | 103020 | Pacific Blue (PB) |
| Cell strainer | BD Falcon | 352340 | 40 µM |
| Collagenase, type IV | Gibco | 17104-019 | |
| cOmplete Mini | Roche | 118 361 700 0 | |
| DAB + Chromogen | Dako | K3468 | |
| Dithiothreitol | Sigma | 43815 | |
| DMEM-F12 | Sigma | D8900 | |
| DNAse I | Roche | 11284932001 | |
| DSP (M-20) Antibody, 1: 100 | Santa Cruz Biotechnology | LS-C20939 | |
| ECC-1 | ATCC | CRL-2923 | Human endometrium adenocarcinoma cell line |
| Epidermal growth factor | Sigma | E9644 | |
| Hepes 0.01 M | Sigma | MFCD00006158 | |
| Fibroblast growth factor basic | Sigma | F0291 | |
| Giemsa Stain, modified GS-500 | Sigma | MFCD00081642 | |
| Glycerol | Dako | C0563 | |
| Haemocytometer | VWR | HERE1080339 | |
| HCC1806 | ATCC | CRL-2335 | Human mammary squamous cell carcinoma cell line |
| Insulin, transferrin, selenium Solution | Gibco | 41400045 | |
| May-Grünwald Stain MG500 | Sigma | MFCD00131580 | |
| MCF7 | ATCC | HTB-22 | Human mammary adenocarcinoma cell line |
| Methylcellulose | AlfaAesar | 45490 | |
| NaCl | JMGS | 37040005002212 | |
| Polyclonal Rabbit Anti-goat immunoglobulins / HRP, 1: 100 | Dako | G-21234 | |
| Poly(2-hydroxyethyl-methacrylate | Sigma | P3932 | |
| Putrescine | Sigma | P7505 | |
| RL95-2 | ATCC | CRL-1671 | Human endometrium carcinoma cell line |
| Sodium deoxycholic acid | JMS | EINECS 206-132-7 | |
| Sodium dodecyl sulfate | Sigma | 436143 | |
| Substrate Buffer | Dako | 926605 | |
| Tris | JMGS | 20360000BP152112 | |
| Triton-X 100 | Merck | 108603 | |
| Trypan blue | Sigma | T8154 | |
| Trypsin-EDTA | Sigma | T4049 | |
| β-actin antibody | Sigma | A5316 |
References
- Williams, D. F. On the mechanisms of biocompatibility. Biomaterials. 29 (20), 2941-2953 (2008).
- Bruinink, A., Luginbuehl, R. Evaluation of biocompatibility using in vitro methods: interpretation and limitations. Advances in Biochemical Engineering/Biotechnology. 126, 117-152 (2012).
- Wataha, J. C. Principles of biocompatibility for dental practitioners. The Journal of Prosthetic Dentistry. 86 (2), 203-209 (2001).
- Mishra, S. F. D. A. CE mark or something else?-Thinking fast and slow. Indian Heart Journal. 69 (1), 1-5 (2016).
- Barbeck, M., et al. Balancing Purification and Ultrastructure of Naturally Derived Bone Blocks for Bone Regeneration: Report of the Purification Effort of Two Bone Blocks. Materials. 12 (19), 3234 (2019).
- Ruzza, P., et al. H-Content Is Not Predictive of Perfluorocarbon Ocular Endotamponade Cytotoxicity in Vitro. ACS Omega. 4 (8), 13481-13487 (2019).
- Coelho, C. C., Araújo, R., Quadros, P. A., Sousa, S. R., Monteiro, F. J. Antibacterial bone substitute of hydroxyapatite and magnesium oxide to prevent dental and orthopaedic infections. Materials Science and Engineering: C. 97, 529-538 (2019).
- Jung, O., et al. Improved In Vitro Test Procedure for Full Assessment of the Cytocompatibility of Degradable Magnesium Based on ISO 10993-5/-12. International Journal of Molecular Sciences. 20 (2), 255 (2019).
- Ruzza, P., et al. H-Content Is Not Predictive of Perfluorocarbon Ocular Endotamponade Cytotoxicity in Vitro. ACS Omega. 4 (8), 13481-13487 (2019).
- ISO. I.O. for S. ISO 10993-12:2012 - part 12: Sample preparation and reference materials. ISO. , (2012).
- ISO. I.O. for S. ISO 10993-5:2009 Biological evaluation of medical devices - part 5: Tests for in vitro cytotoxicity. ISO. , (2009).
- Srivastava, G. K., et al. Comparison between direct contact and extract exposure methods for PFO cytotoxicity evaluation. Scientific Reports. 8 (1), 1425 (2018).
- Pusnik, M., Imeri, M., Deppierraz, G., Bruinink, A., Zinn, M. The agar diffusion scratch assay--A novel method to assess the bioactive and cytotoxic potential of new materials and compounds. Scientific Reports. 6, 20854 (2016).
- Spiller, K. L., et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials. 35 (15), 4477-4488 (2014).
- Zhou, H., et al. In Vitro Cytotoxicity Evaluation of a Novel Root Repair Material. Journal of Endodontics. 39 (4), 478-483 (2013).
- Bordron, A., et al. The binding of some human antiendothelial cell antibodies induces endothelial cell apoptosis. Journal of Clinical Investigation. 101 (10), 2029-2035 (1998).
- Palmini, G., et al. Establishment of Cancer Stem Cell Cultures from Human Conventional Osteosarcoma. Journal of Visualized Experiments. (116), e53884 (2016).
- Gregory, C. A., Grady Gunn, W., Peister, A., Prockop, D. J. An Alizarin red-based assay of mineralization by adherent cells in culture: comparison with cetylpyridinium chloride extraction. Analytical Biochemistry. 329 (1), 77-84 (2004).
- Cai, S., Zhang, W., Chen, W. PDGFRβ+/c-kit+ pulp cells are odontoblastic progenitors capable of producing dentin-like structure in vitro and in vivo. BMC Oral Health. 16 (1), 113 (2016).
- Paula, A., et al. Biodentine Boosts, WhiteProRoot MTA Increases and Life Suppresses Odontoblast Activity. Materials. 12 (7), 1184 (2019).
- Chander, N. G. Standardization of in vitro studies. Journal of Indian Prosthodontic Society. 16 (3), 227-228 (2016).
- Cavalcanti, B. N., Rode de M, S., França, C. M., Marques, M. M. Pulp capping materials exert an effect on the secretion of IL-1β and IL-8 by migrating human neutrophils. Brazilian Oral Research. 25 (1), 13-18 (2011).
- Chang, S., Lee, S. Y., Ann, H. J., Kum, K. Y., Kim, E. C. Effects of calcium silicate endodontic cements on biocompatibility and mineralization-inducing potentials in human dental pulp cells. Journal of Endodontics. 40 (8), 1194-1200 (2014).
- Daltoé, M. O., Paula-Silva, F. W. G., Faccioli, L. H., Gatón-Hernández, P. M., De Rossi, A., Bezerra Silva, L. A. Expression of Mineralization Markers during Pulp Response to Biodentine and Mineral Trioxide Aggregate. Journal of Endodontics. 42 (4), 596-603 (2016).
- Elias, R. V., Demarco, F. F., Tarquinio, S. B. C., Piva, E. Pulp responses to the application of a self-etching adhesive in human pulps after controlling bleeding with sodium hypochlorite. Quintessence International. 38 (2), 67-77 (2007).
- Huang, G. T. J., Shagramanova, K., Chan, S. W. Formation of odontoblast-like cells from cultured human dental pulp cells on dentin in vitro. Journal of endodontics. 32 (11), 1066-1073 (2006).
- Jafarnia, B., et al. Evaluation of cytotoxicity of MTA employing various additives. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 107 (5), 739-744 (2009).
- Paranjpe, A., Smoot, T., Zhang, H., Johnson, J. D. Direct contact with mineral trioxide aggregate activates and differentiates human dental pulp cells. Journal of Endodontics. 37 (12), 1691-1695 (2011).
- Spagnuolo, G., et al. In vitro cellular detoxification of triethylene glycol dimethacrylate by adduct formation with N-acetylcysteine. Dental Materials. 29 (8), 153-160 (2013).
- Murray, P. E., García Godoy, C., García Godoy C, F. How is the biocompatibilty of dental biomaterials evaluated. Medicina Oral, Patologia Oral y Cirugia Bucal. 12 (3), 258-266 (2007).
- Hanks, C. T., Wataha, J. C., Sun, Z. In vitro models of biocompatibility: a review. Dental Materials. 12 (3), 186-193 (1996).
- Eid, A. A., et al. In Vitro Biocompatibility and Oxidative Stress Profiles of Different Hydraulic Calcium Silicate Cements. Journal of Endodontics. 40 (2), 255-260 (2014).
- Nocca, G., et al. Effects of ethanol and dimethyl sulfoxide on solubility and cytotoxicity of the resin monomer triethylene glycol dimethacrylate. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 100 (6), 1500-1506 (2012).
- Abuarqoub, D., Aslam, N., Jafar, H., Abu Harfil, Z., Awidi, A. Biocompatibility of Biodentine with Periodontal Ligament Stem Cells: In Vitro Study. Dentistry Journal. 8 (1), 17 (2020).
- Coelho, A. S., et al. Cytotoxic effects of a chlorhexidine mouthwash and of an enzymatic mouthwash on human gingival fibroblasts. Odontology. 108 (2), 260-270 (2020).
- Wang, M. O., et al. Evaluation of the In Vitro Cytotoxicity of Cross-Linked Biomaterials. Biomacromolecules. 14 (5), 1321-1329 (2013).
- Tyliszczak, B., Drabczyk, A., Kudłacik-Kramarczyk, S., Bialik-Wąs, K., Sobczak-Kupiec, A. In vitro cytotoxicity of hydrogels based on chitosan and modified with gold nanoparticles. Journal of Polymer Research. 24 (10), 153 (2017).
- Widbiller, M., et al. Three-dimensional culture of dental pulp stem cells in direct contact to tricalcium silicate cements. Clinical Oral Investigations. 20 (2), 237-246 (2016).
- Pintor, A. V. B., et al. In Vitro and In Vivo Biocompatibility of ReOss in Powder and Putty Configurations. Brazilian Dental Journal. 29 (2), 117-127 (2018).
- Pellissari, C. V. G., et al. In Vitro Toxic Effect of Biomaterials Coated with Silver Tungstate or Silver Molybdate Microcrystals. Journal of Nanomaterials. 2020, 1-9 (2020).
- Collado-González, M., et al. Cytotoxicity and bioactivity of various pulpotomy materials on stem cells from human exfoliated primary teeth. International Endodontic Journal. 50, 19-30 (2017).
- Paula, A., et al. Direct Pulp Capping: Which is the Most Effective Biomaterial? A Retrospective Clinical Study. Materials. 12 (20), 3382 (2019).
- Williams, D. F. There is no such thing as a biocompatible material. Biomaterials. 35 (38), 10009-10014 (2014).
- Schuh, J. C. L. Medical device regulations and testing for toxicologic pathologists. Toxicologic Pathology. 36 (1), 63-69 (2008).
- Pizzoferrato, A., et al. Cell culture methods for testing Biocompatibility. Clinical Materials. 15 (3), (1994).
- Pereira Paula, A. B., et al. Direct pulp capping: what is the most effective therapy? - review and meta-analysis. Journal of Evidence Based Dental Practice. , (2018).
- Caiaffa, K. S., et al. Effect of analogues of cationic peptides on dentin mineralization markers in odontoblast-like cells. Archives of Oral Biology. 103, 19-25 (2019).
- Fujiwara, S., Kumabe, S., Iwai, Y. Isolated rat dental pulp cell culture and transplantation with an alginate scaffold. Okajimas Folia Anatomica Japonica. 83 (1), 15-24 (2006).
- Nakashima, M., et al. Stimulation of Reparative Dentin Formation by Ex Vivo Gene Therapy Using Dental Pulp Stem Cells Electrotransfected with Growth/differentiation factor 11 (Gdf11). Human Gene Therapy. 15 (11), 1045-1053 (2004).
- Narayanan, K., et al. Differentiation of embryonic mesenchymal cells to odontoblast-like cells by overexpression of dentin matrix protein 1. Proceedings of the National Academy of Sciences of the United States of America. 98 (8), 4516-4521 (2001).
- Kim, H. J., Yoo, J. H., Choi, Y., Joo, J. Y., Lee, J. Y., Kim, H. J. Assessing the effects of cyclosporine A on the osteoblastogenesis, osteoclastogenesis, and angiogenesis mediated by the human periodontal ligament stem cells. Journal of Periodontology. , (2019).
- Bou Assaf, R., et al. Healing of Bone Defects in Pig's Femur Using Mesenchymal Cells Originated from the Sinus Membrane with Different Scaffolds. Stem Cells International. , (2019).
- He, W., et al. Lipopolysaccharide enhances decorin expression through the toll-like receptor 4, myeloid differentiating factor 88, nuclear factor-kappa B, and mitogen-activated protein kinase pathways in odontoblast cells. Journal of Endodontics. 38 (4), 464-469 (2012).
- Xiong, Y., et al. Wnt Production in Dental Epithelium Is Crucial for Tooth Differentiation. Journal of Dental Research. 98 (5), 580-588 (2019).
- Haruyama, N., et al. Genetic evidence for key roles of decorin and biglycan in dentin mineralization. Matrix Biology. 28 (3), 129-136 (2009).
- Sreenath, T., et al. Dentin Sialophosphoprotein Knockout Mouse Teeth Display Widened Predentin Zone and Develop Defective Dentin Mineralization Similar to Human Dentinogenesis Imperfecta Type III. Journal of Biological Chemistry. 278 (27), 24874-24880 (2003).
- Yang, Y., Zhao, Y., Liu, X., Chen, Y., Liu, P., Zhao, L. Effect of SOX2 on odontoblast differentiation of dental pulp stem cells. Molecular Medicine Reports. 16 (6), 9659-9663 (2017).
- Tao, H., et al. Klf4 Promotes Dentinogenesis and Odontoblastic Differentiation via Modulation of TGF-β Signaling Pathway and Interaction With Histone Acetylation. Journal of Bone and Mineral Research. 34 (8), 1502-1516 (2019).
- Massa, L. F., Ramachandran, A., George, A., Arana-Chavez, V. E. Developmental appearance of dentin matrix protein 1 during the early dentinogenesis in rat molars as identified by high-resolution immunocytochemistry. Histochemistry and Cell Biology. 124 (3-4), 197-205 (2005).
- Hao, J., Zou, B., Narayanan, K., George, A. Differential expression patterns of the dentin matrix proteins during mineralized tissue formation. Bone. 34 (6), 921-932 (2004).
- Tompkins, K., Alvares, K., George, A., Veis, A. Two related low molecular mass polypeptide isoforms of amelogenin have distinct activities in mouse tooth germ differentiation in vitro. Journal of Bone and Mineral Research. 20 (2), 341-349 (2005).
- Zhai, Y., et al. Activation and Biological Properties of Human β Defensin 4 in Stem Cells Derived From Human Exfoliated Deciduous Teeth. Frontiers in Physiology. 10, (2019).
- Bègue-Kirn, C., Ruch, J. V., Ridall, A. L., Butler, W. T. Comparative analysis of mouse DSP and DPP expression in odontoblasts, preameloblasts, and experimentally induced odontoblast-like cells. European Journal of Oral Sciences. 106, 254-259 (1998).
- Kikuchi, H., Suzuki, K., Sakai, N., Yamada, S. Odontoblasts induced from mesenchymal cells of murine dental papillae in three-dimensional cell culture. Cell and Tissue Research. 317 (2), 173-185 (2004).
- Li, X., Yang, G., Fan, M. Effects of homeobox gene distal-less 3 on proliferation and odontoblastic differentiation of human dental pulp cells. Journal of Endodontics. 38 (11), 1504-1510 (2012).
- Chen, S., et al. Differential regulation of dentin sialophosphoprotein expression by Runx2 during odontoblast cytodifferentiation. Journal of Biological Chemistry. 280 (33), 29717-29727 (2005).
- Narayanan, K., Gajjeraman, S., Ramachandran, A., Hao, J., George, A. Dentin matrix protein 1 regulates dentin sialophosphoprotein gene transcription during early odontoblast differentiation. Journal of Biological Chemistry. 281 (28), 19064-19071 (2006).
- Buchaille, R., Couble, M. L., Magloire, H., Bleicher, F. A substractive PCR-based cDNA library from human odontoblast cells: identification of novel genes expressed in tooth forming cells. Matrix Biology. 19 (5), 421-430 (2000).
- Miyazaki, T., Baba, T., Mori, T., Komori, T. Collapsin Response Mediator Protein 1, a Novel Marker Protein for Differentiated Odontoblasts. Acta Histochemica et Cytochemica. 51 (6), 185-190 (2018).
- Yokoi, M., Kuremoto, K., Okada, S., Sasaki, M., Tsuga, K. Effect of attenuation of fibroblast growth factor receptor 2b signaling on odontoblast differentiation and dentin formation. In Vitro Cellular and Developmental Biology - Animal. 55 (3), 211-219 (2019).
- Tohma, A., et al. Glucose Transporter 2 and 4 Are Involved in Glucose Supply during Pulpal Wound Healing after Pulpotomy with Mineral Trioxide Aggregate in Rat Molars. Journal of Endodontics. , (2019).
- Sueyama, Y., Kaneko, T., Ito, T., Kaneko, R., Okiji, T. Implantation of Endothelial Cells with Mesenchymal Stem Cells Accelerates Dental Pulp Tissue Regeneration/Healing in Pulpotomized Rat Molars. Journal of Endodontics. 43 (6), 943-948 (2017).
- Petersson, U., Hultenby, K., Wendel, M. Identification, distribution and expression of osteoadherin during tooth formation. European Journal of Oral Sciences. 111 (2), 128-136 (2003).
- Couble, M. L., et al. Immunodetection of osteoadherin in murine tooth extracellular matrices. Histochemistry and Cell Biology. 121 (1), 47-53 (2004).
- Buchaille, R., Couble, M. L., Magloire, H., Bleicher, F. Expression of the small leucine-rich proteoglycan osteoadherin/osteomodulin in human dental pulp and developing rat teeth. Bone. 27 (2), 265-270 (2000).
- Salmon, B., et al. Abnormal osteopontin and matrix extracellular phosphoglycoprotein localization, and odontoblast differentiation, in X-linked hypophosphatemic teeth. Connective Tissue Research. 55, 79-82 (2014).
- Liao, C., Ou, Y., Wu, Y., Zhou, Y., Liang, S., Wang, Y. Sclerostin inhibits odontogenic differentiation of human pulp-derived odontoblast-like cells under mechanical stress. Journal of Cellular Physiology. 234 (11), 20779-20789 (2019).
- Deng, X., et al. The combined effect of oleonuezhenide and wedelolactone on proliferation and osteoblastogenesis of bone marrow mesenchymal stem cells. Phytomedicine. 153103, (2019).
- Choi, H., Kim, T. H., Yun, C. Y., Kim, J. W., Cho, E. S. Testicular acid phosphatase induces odontoblast differentiation and mineralization. Cell and Tissue Research. 364 (1), 95-103 (2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved