A subscription to JoVE is required to view this content. Sign in or start your free trial.
Methods Article
Leveraging Turbidity and Thromboelastography for Complementary Clot Characterization
In This Article
Summary
Fibrin is responsible for clot formation during hemostasis and thrombosis. Turbidity assays and thromboelastograhy (TEG) can be utilized as synergistic tools that provide complementary assessment of a clot. These two techniques together can give more insight into how clotting conditions affect fibrin clot formation.
Abstract
Thrombosis is a leading cause of death worldwide. Fibrin(ogen) is the protein primarily responsible for clot formation or thrombosis. Therefore, characterizing fibrin clot formation is beneficial to the study of thrombosis. Turbidity and thromboelastography (TEG) are both widely utilized in vitro assays for monitoring clot formation. Turbidity dynamically measures the light transmittance through a fibrin clot structure via a spectrometer and is often used in research laboratories. TEG is a specialized viscoelastic technique that directly measures blood clot strength and is primarily utilized in clinical settings to assess patients' hemostasis. With the help of these two tools, this study describes a method for characterizing an in vitro fibrin clot using a simplified fibrinogen/thrombin clot model. Data trends across both techniques were compared under various clotting conditions. Human and bovine fibrin clots were formed side-by-side in this study as bovine clotting factors are often used as substitutes to human clotting factors in clinical and research settings. Results demonstrate that TEG and turbidity track clot formation via two distinct methods and when utilized together provide complementary clot strength and fiber structural information across diverse clotting conditions.
Introduction
Thrombosis is the pathological formation of a blood clot in the body that blocks blood circulation leading to high morbidity and mortality worldwide. There are 1 to 2 cases of venous thromboembolism and 2 to 3 cases of thrombosis-induced vascular diseases per 1000 people annually1,2. Presented here is a method leveraging thromboelastography (TEG) and turbidity to monitor clot formation under various clotting conditions. Fibrin(ogen) is the primary protein that is responsible for clot formation in the body. In the final steps of the coagulation cascade, fibrinopeptides are cleaved from fibrinogen by thrombin initiating the polymerization of insoluble fibrin monomers as the clot develops3,4. To understand clot formation in pathological thrombosis, it is necessary to characterize fibrin formation under diverse clotting circumstances. Multiple clot monitoring assays have been utilized to study fibrin clot formation in vitro. Prothrombin time (PT/INR) and activated partial thromboplastin time (aPTT) are two common clinical assays that measure the integrity of a specific coagulation pathway. However, they use time as the only variable that gives no indication of physical clot properties5. Electron microscopy allows visualization of the micro-structure of a completely formed fibrin clot but provides no information about the clot forming process itself6. Among all assays, turbidity assays and TEG offer the ability to track clot characteristics dynamically over time. These techniques enable the measure of comprehensive clotting profiles and therefore, provide some benefit over other fibrin clot characterization tools.
Specifically, turbidity assays (or clot turbidimetry) is widely used for research and clinical applications due to its simplistic implementation and the wide accessibility of spectrometers in research laboratories. This assay allows a dynamic measurement of light transmittance through a forming clot by taking individual repetitive readings at a defined wavelength (most commonly at a wavelength in the range of 350 – 700 nm)7. Temperature in the reading chamber can also be adjusted. As fibrin gel forms, the amount of light that travels through the protein network is reduced causing an increase in absorbance over time. Similarly, absorbance decreases when the clot network degrades. Turbidity assays can easily be multiplexed using a multi-well plate format to allow for high throughput sample screening in both 96- and 384-well plates. Several clot characteristics can be derived from a turbidity tracing curve (absorbance over time measurement) that include: maximum turbidity, time to maximum turbidity, time to clot onset, and clot formation rate (Vmax). A fibrin fiber mass/length ratio can also be derived from raw turbidity data to estimate fibrin fiber thickness8,9,10.
TEG is primarily utilized in the clinical setting to assess patients’ hemostasis and clot lysis. It is also commonly used in surgical applications to determine when anti-fibrinolytic drugs or hemostatic blood products should be administered11,12. Clot formation occurs inside a TEG cup with all the clotting components being added to the cup prior to the initiation of the assay. The cup, with evolving clot, physically rotates against a pin that is inserted into its center and an electromechanical torsion sensor measures the increasing viscoelastic strength of the clot. This assay is typically carried out at the physiological temperature of 37 °C; however, the temperature can be manually adjusted on the instrument. Maximum amplitude (MA), reaction rate (R), kinetics time (K), α-angle (Angle), and time to maximum amplitude (TMA) are extracted by the TEG software from the dynamic TEG tracing. These values are typically compared with clinical normal ranges to assess a patient’s coagulation state. While TEG is not precisely a viscometer, as it measures clot strength in millimeter units, it does provide important viscoelastic clot data and functions as a valuable clinical decision making tool for physicians to decide to administer specific blood products and adjust therapeutic dosing13. When both TEG and turbidity assays are utilized together, they provide complementary clot characterization information as clot strength and kinetics are easily extracted from TEG and fibrin fiber thickness can be accessed by optical turbidity measurements.
As fibrin is a critical component of a blood clot, fibrin clot characterization under diverse clot formation conditions can provide valuable insight into how a specific variable contributes to the clot formation process and ultimate clot properties. Understanding this can provide guidance for thrombosis diagnosis and the development of therapeutics. To obtain a more representative fibrin clot characterization, plasma can be substituted to monitor clot formation as it resembles in vivo clotting conditions more closely than a simplified fibrinogen/thrombin model system. However, due to the intricate nature of the coagulation cascade, clot formation using plasma adds to the complexity, making it more difficult to isolate the impact of individual factors. Utilizing a simplified fibrinogen/thrombin model prevents the need to initiate the entire clotting cascade allowing for isolation of the final fibrin formation step. By including two major fibrin forming components (fibrinogen and thrombin), this setup creates a highly controlled clot formation condition. It is also important to note that while the simplified clot model is used here, this protocol can also be utilized to characterize more complex clots by including additional clotting factors. In this study, fibrin clot characterization using turbidity and TEG are carried out by varying fibrinogen and thrombin concentrations, ionic strength, pH, and total protein concentration in the clotting solution to mimic different in vivo clotting circumstances14. Details regarding these variations to the protocol have been included in Section 5.
Protocol
1. Preparation of phosphate buffer saline (PBS)
NOTE: PBS was used throughout this study as the described assays did not require the addition of calcium. It is important to note that when adding calcium, often utilized to re-calcify citrated blood products, PBS should be avoided as calcium is known to precipitate in phosphate buffers.
- Make a 0.01 M, pH 7.4 PBS buffer by mixing 137 mM sodium chloride, 1.8 mM potassium phosphate monobasic, 10 mM sodium phosphate dibasic and 2.7 mM potassium chloride in DI water.
- Verify buffer pH using a pH probe and adjust the pH using sodium hydroxide or hydrochloric acid as needed.
- Use this PBS for preparation of fibrinogen and clotting assays (unless otherwise specified).
NOTE: Buffer is suggested to reconstitute fibrinogen powder since rehydration in DI water can result in fibrinogen precipitation even at 37 °C.
2. Preparation and storage of proteins
NOTE: Throughout the protocol the protein stock concentrations are prepared at different concentrations for turbidity and TEG to allow for the consistent ratio of salt, DI water, PBS and other residual factors in the final clotting solutions.
- Preparation and storage of fibrinogen
NOTE: Contaminants in commercially available fibrinogen include a significant amount of factor XIII, residual amounts of other clotting factors, storage buffer and salts. In the presence of calcium, factor XIII is known to crosslink the fibrin clot network. This effect contributes to a tightened clot structure and enhanced clot strength affecting fibrin characterization. Commercially available activity kits can be used to determine active factor XIIIa levels. To minimize variability caused by factor XIIIa, experiments should be designed with the exclusion of calcium or additional steps to remove factor XIIIa should be incorporated into this protocol. In addition, protein storage salt and buffer type should be assessed and if necessary dialysis can be carried out to transfer to a preferred assay working buffer.- Weigh and aliquot lyophilized fibrinogen powder (bovine or human) in 2 mL tubes at 20 mg of protein per tube and store aliquots at -20 °C for up to 6 months.
- Allow fibrinogen aliquot to acclimate to RT (room temperature) for 10 minutes on the day of the experiment. Reconstitute fibrinogen by adding 600 µL of PBS to the aliquot 20 min prior to use.
- Take 10 µL of fibrinogen and dilute it with 190 µL of PBS in a UV transparent 96-well plate or a cuvette. Determine fibrinogen concentration by taking absorbance at 280 nm via a commercial spectrometer and its software.
- Calculate fibrinogen concentration (mg/mL) using Beer’s law. Prepare 12 mg/mL (for turbidity assays) and 3.2 mg/mL (for TEG) fibrinogen stock solutions by further dilution with PBS (unless otherwise specified).
NOTE: Fibrinogen concentration determination (mg/mL) by Beer’s law:
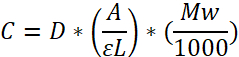
Molar extinction coefficient: ɛ = 513,400 L mol-1 cm-1 at 280 nm; Pathlength (L); Dilution factor (D); Molecular Weight (MW) = 340,000 Da. ɛ is derived by multiplying E0.1% = 1.51 (280 nm) (extinction coefficient, given by the supplier) with MW.
- Preparation and storage of thrombin
- Reconstitute lyophilized thrombin (bovine or human, 1000 U stock) in 200 µL of deionized (DI) water to make 200 µL of 5000 U/mL thrombin stock solution.
- Make 5 µL (20 U/tube) aliquots of the solution and keep aliquots frozen at −20 °C.
- Thaw thrombin aliquots at RT for 15 min on the day of experiment and make thrombin working solution by diluting it to 20 U/mL for turbidity assays and 18 U/mL for TEG with DI water (unless otherwise specified).
NOTE: Precautions should be taken to maintain enzyme activity which can be accomplished by maintaining enzymes on ice during thawing and use; however, no reduction in thrombin activity was observed when utilized directly after thawing at RT.
3. Turbidity
- Use any commercially available spectrometer that has an absorbance range of 350 - 700 nm and a corresponding software to monitor clot turbidity over time (see Table of Materials).
- Turn on the spectrometer and open the corresponding analysis software.
- Select plate 1 and open plate settings tab. Click ABS mode and Kinetic to monitor a dynamic absorbance reading over time.
- Select 550 nm (or any value in the range of 350 – 700 nm) in the wavelength tab and adjust the total run time to be 60 min with an interval of 30 s in the timing tab. Select wells of interest for reading by highlighting the wells. Adjust other settings if needed.
NOTE: Selecting a wavelength at the lower end of the range (around 350 nm) brings a better sensitivity but absorbance might also exceed the detection limit of the spectrometer. The most commonly utilized wavelength for turbidity measurement is 405 nm in literature; however, this protocol uses 550 nm to ensure the dynamic turbidity values are within the detection limit for all experiments. The selected reading interval should be as short as possible to achieve the highest level of assay sensitivity. This will depend upon the spectrometer and number of wells being read during a given assay. - Take a UV transparent 96-well plate. Pipette 140 µL PBS in a well that is selected for reading. Add and mix 10 µL of thrombin (20 U/mL) in the well.
NOTE: Do not use high binding assay plates to minimize nonspecific protein binding to the well surface. This could affect protein dispersion in the solution and result in high assay variability. - Initiate clotting immediately by adding 50 µL of fibrinogen (12 mg/mL) in the well to obtain a 200 µL clotting solution with a final concentration of 3 mg/mL fibrinogen and 1 U/mL thrombin. Mix the contents in the well by pipetting up and down five times taking care to avoid the creation of bubbles in the solution as they will impact absorbance by scattering light.
NOTE: Use a multichannel pipette when running multiple clot samples on the same plate at the same time. Record time differences across wells and the time period prior to the first read by the instrument to offset clotting times. - Place the 96-well plate in the holder and click Start in the software to start turbidity reading at RT.
NOTE: If carrying out the assay at an elevated temperature then the spectrometer, plate, and reagents must all be maintained at the desired temperature prior to clot initiation. - Once completed, retrieve the turbidity data and obtain a turbidity tracing curve by plotting absorbance change over time in a plotting software.
- Derive TurbMax (maximum turbidity indicative of fibrin fiber thickness and fibrin network density) by taking the max absorbance value of the curve over time.
NOTE: Fibrin fiber mass/length ratio can be calculated from turbidity values using the equation provided in the following manuscript8. - Calculate 90% maximum turbidity by multiplying TurbMax by 90%. Derive TurbTime by computing time from clot initiation to 90% maximum turbidity.
NOTE: The time to 90% maximum turbidity is a more reliable metric than time to absolute maximum turbidity as it better represents clot time by eliminating the highly variable final clot formation period. Additional clotting parameters such as clot onset time (time from start of test to when absorbance starts to increase), and clot formation rate (Vmax, the largest slope of the linear region in the turbidity tracing curve) can also be extracted from the turbidity tracings.
4. Thromboelastography (TEG)
- Turn on the thromboelastograph analyzer and wait for the temperature to stabilize at 37 °C.
- Open TEG - software. Once logged in, create an experiment name under the ID section.
- Conduct an e-test for all channels by following the on-screen software prompts. Place the lever back to the load position once all checks are complete.
NOTE: A TEG e-test is required and should be conducted every time when using the instrument. TEG coagulation control assays (using TEG level 1 and level 2 controls) are required at the regular manufacturer suggested intervals when utilized for clinical samples. - Click the TEG tab, input sample information for channels that will be used. Place a clear uncoated TEG cup in its corresponding channel. Slide the carrier up to the top and press the cup bottom 5-times to affix the pin to the torsion rod. Lower the carrier and press the cup downward into the carrier base until it “clicks”.
- Pipette 20 µL of thrombin solution (18 U/mL) into the TEG cup. Initiate clotting immediately by adding 340 µL of fibrinogen (3.2 mg/mL) into the TEG cup to obtain a 360 µL clotting solution with a final concentration of 3 mg/mL fibrinogen and 1 U/mL thrombin in the cup. Mix the contents by pipetting up and down five times.
NOTE: Potential clotting factors or other components of interest should be added during this step being careful to always maintain a final volume of 360 µL in the TEG cup. - Slide the cup loaded carrier up, move the lever to the read position and click Start in the software to initiate the TEG reading.
- Once TEG is completed (after about an hour), retrieve TEG parameters and obtain a TEG tracing curve by plotting amplitude over time in a plotting software.
- Collect MA as TEGMax (maximum amplitude is indicative of clot strength) and TMA as TEGTime (time to maximum amplitude) from the software.
NOTE: MA is calculated by the software as the maximum amplitude at the time at which the amplitude has less than a 5% deviation over a 3-minute period of time. TMA is determined as the time from the maximum thrombus generation rate (near the split point) to the MA. Other parameters might also be useful to assess when performing the clot analysis. Some examples of these parameters include: R-time (the time from start of test to when amplitude reaches 2 mm), K (the time from the end of R to when amplitude reaches 20 mm), alpha (slope of line between R and K), and CLT (clot lysis time).
5. Fibrin characterization under different clotting conditions
NOTE: Perform fibrin characterization experiments by modulating a specific variable in the clotting solutions such as: fibrinogen and thrombin concentrations, ionic strength, pH, and total protein concentrations. Experimental preparations with these example variables are described in this section; however, other clotting factors and conditions of interest can be substituted as well. Carefully select a suitable buffer system taking into consideration each unique assay requirements. For turbidity and TEG assays, include a buffer only control to ensure an accurate background subtraction while analyzing the effect of these variables.
- Varying fibrinogen concentration (1, 2, 3, 4, 5 mg/mL)
- Adjust step 2.1.4 to prepare the fibrinogen stock at different concentrations (4, 8, 12, 16, 20 mg/mL for turbidity assays and 1.1, 2.1, 3.2, 4.2, 5.3 mg/mL for TEG).
- Adjust step 3.6 to “add 50 µL fibrinogen (4, 8, 12, 16, 20 mg/mL) into multiple wells of the 96 well-plate” for turbidity assays.
- Adjust step 4.5 to “add 340 µL fibrinogen (1.1, 2.1, 3.2, 4.2, 5.3 mg/mL) into clear TEG cups” for TEG.
- Varying thrombin concentration (0.1, 0.3, 0.6, 0.8, 1, 2.5, 5, 10 U/mL)
- Adjust step 2.2.3 to prepare thrombin stock at different concentrations (2, 6, 12, 16, 20, 50, 100, 200 U/mL for turbidity assays and 1.8, 5.4, 10.8, 14.4, 18, 45, 90, 180 U/mL for TEG).
- Adjust step 3.5 to “add 10 µL thrombin (2, 6, 12, 16, 20, 50, 100, 200 U/mL) into multiple wells of the 96 well-plate” for turbidity assays.
- Adjust step 4.5 to “pipette 20 µL thrombin (1.8, 5.4, 10.8, 14.4, 18, 45, 90, 180 U/mL) into clear TEG cups” for TEG.
- Varying ionic strength (0.05, 0.13, 0.14, 0.15, 0.16, 0.17 and 0.3 M)
- Dissolve sodium chloride (21, 101, 111, 121, 131, 141, and 271 mM) along with 1.8 mM potassium phosphate monobasic, 10 mM sodium phosphate dibasic and 2.7 mM potassium chloride in DI water to make 0.01 M PBS solutions with varying ionic strengths.
- Adjust step 1.3 to use PBS made at different ionic strengths to prepare fibrinogen and clotting solutions for both turbidity and TEG assays.
- Varying pH (5.8, 6.6, 7.3, 7.4, 7.5, and 8.0)
- Dissolve sodium phosphate dibasic (0.7, 3.2, 7.7, 8.1, 8.4, 9.5 mM) and potassium phosphate monobasic (8.2, 6.0, 2.0, 1.7, 1.4, 0.5 mM) along with 2.7 mM potassium chloride and sodium chloride (153, 147, 138, 137, 136, 134 mM) to make 0.01 M PBS solutions with varying pH and a final ionic strength at 0.165 M.
- Verify buffer pH value via pH probe and adjust pH if needed.
- Adjust step 1.3 to use PBS made at different pH to prepare fibrinogen and clotting solution for both turbidity and TEG assays.
- Varying albumin concentration (0, 20, 40, 50, 60, 80, 100 mg/mL)
- Dissolve 2 g of lyophilized albumin in 500 µL of PBS at RT for 20 min on the day of experiment.
- Determine albumin concentration using the same procedure mentioned in step 2.1.3 and 2.1.4 with a molar extinction coefficient of 43,800 L mol−1 cm−1 (at 280 nm) for albumin.
- Prepare albumin stock at different concentrations.
- Adjust step 3.5 to “Pipette 40 µL PBS in a well that is selected for reading and add 10 µL thrombin (20 U/mL)”. Adjust step 3.6 to “Initiate clotting immediately by adding 50 µL fibrinogen (12 mg/mL) with 100 µL albumin (0, 40, 80, 100, 120, 160, 200 mg/mL) into multiple wells to a final concentration of 3 mg/mL fibrinogen, 1 U/mL thrombin and 0, 20, 40, 50, 60, 80, 100 mg/mL albumin in wells.”
- Adjust step 4.5 to “Initiate clotting immediately by adding a mixture of 200 µL albumin (36, 72, 90, 108, 144, 180 mg/mL) and 140 µL 7.7 mg/mL fibrinogen into TEG cups to obtain a 360 µL clotting solution with a final concentration of 3 mg/mL fibrinogen, 1 U/mL thrombin and 0, 20, 40, 50, 60, 80, 100 mg/mL albumin in TEG cups.”
Results
The experiments shown in Figure 1 are representative turbidity tracing curves of human and bovine fibrin clots at different fibrinogen levels. Representative TEG tracing curves for fibrin clot formation at different fibrinogen levels are shown in Figure 2. Both tracing curves demonstrate that after a lag period following clot initiation, clot turbidity or clot amplitude increases over time and levels off at the end of clot formation. An endpoint value of maximum...
Discussion
This protocol demonstrates the utilization of two distinct clot characterization tools testing a simplified fibrinogen/thrombin clot model using commercially available components. Both TEG and turbidity assays are easy to conduct. They not only provide end point clot examinations such as max clot formation (TurbMax and TEGMax) and clot formation times (TurbTime and TEGTime) but also assess the dynamic clot forming process. This makes TEG and turbidity valuable tools for clot ch...
Disclosures
The authors have nothing to disclose.
Acknowledgements
None.
Materials
| Name | Company | Catalog Number | Comments |
| 96-Well Clear Flat Bottom UV-Transparent Microplate | Corning | 3635 | Non-treated acrylic copolymer, non-sterile |
| Albumin from human serum | Millipore Sigma | A1653 | ≥96%, lyophilized powder |
| Arium Mini Plus Ultrapure Water System | Sartorius | NA | DI water source |
| Bovine serum albumin | Millipore Sigma | A2153 | ≥96%, lyophilized powder |
| Disposable Cups and Pins for TEG 5000 (Clear) | Haemonetics | REF 6211 | |
| Fibrinogen, Bovine Plasma | Millipore Sigma | 341573 | contains more than 95% clottable protein |
| Fibrinogen, Plasmingogen-Depleted, Human Plasma | Millipore Sigma | 341578 | Contains ≥ 95% clottable proteins. |
| Phosphate buffered saline | Millipore Sigma | P3813 | Powder, pH 7.4, for preparing 1 L solutions |
| Potassium chloride | Millipore Sigma | 60130 | ≥99.5% purity |
| Potassium phosphate monobasic | Millipore Sigma | P9791 | ≥98% purity |
| SevenEasy pH Meter | Mettler Toledo | S20 | |
| Sodium chloride | Millipore Sigma | 71378 | ≥99.5% purity |
| Sodium phosphate dibasic | Millipore Sigma | 71636 | ≥99.5% purity |
| SpectraMax M5 multi-detection microplate reader system | Molecular Devices | M5 | |
| TEG 5000 Thrombelastograph Hemostasis analyzer system | Haemonetics | 07-022 | |
| Thrombin, Bovine | Millipore Sigma | 605157 | |
| Thrombin, Human Plasma, High Activity | Millipore Sigma | 605195 |
References
- Beckman, M. G., Hooper, W. C., Critchley, S. E., Ortel, T. L. Venous Thromboembolism. A Public Health Concern. American Journal of Preventive Medicine. 38, 495-501 (2010).
- Goldhaber, S. Z., Bounameaux, H. Pulmonary embolism and deep vein thrombosis. The Lancet. 379 (9828), 1835-1846 (2012).
- Weisel, J. W., Litvinov, R. I. Mechanisms of fibrin polymerization and clinical implications. Blood. 121 (10), 1712-1719 (2013).
- Weisel, J. W. Fibrin assembly. Lateral aggregation and the role of the two pairs of fibrinopeptides. Biophysical Journal. 50 (6), 1079-1093 (1986).
- Tripathi, M. M., et al. Clinical evaluation of whole blood prothrombin time (PT) and international normalized ratio (INR) using a Laser Speckle Rheology sensor. Scientific Reports. 7 (1), 1-8 (2017).
- Ryan, E. A., Mockros, L. F., Weisel, J. W., Lorand, L. Structural origins of fibrin clot rheology. Biophysical Journal. 77 (5), 2813-2826 (1999).
- Carr, M. E., Hermans, J. Size and Density of Fibrin Fibers from Turbidity. Macromolecules. 11 (1), 46-50 (1978).
- Carr, M. E., Shen, L. L., Hermans, J. A. N., Chapel, H. . Mass-Length Ratio of Fibrin Fibers from Gel Permeation and Light Scattering. 16, 1-15 (1977).
- Gabriel, D. A., Muga, K., Boothroyd, E. M. . The Effect of Fibrin Structure on Fibrinolysis. , 24259-24263 (1992).
- Wolberg, A. S., Gabriel, D. A., Hoffman, M. Analyzing fibrin clot structure using a microplate reader. Blood Coagulation and Fibrinolysis. 13 (6), 533-539 (2002).
- da Luz, L. T., Nascimento, B., Rizoli, S. Thrombelastography (TEG): practical considerations on its clinical use in trauma resuscitation. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine. 21 (1), 29 (2013).
- Whitten, C. W., Greilich, P. E. Thromboelastography: past, present, and future . Anesthesiology: The Journal of the American Society of Anesthesiologists. 92 (5), 1223-1225 (2000).
- Ranucci, M., Laddomada, T., Ranucci, M., Baryshnikova, E. Blood viscosity during coagulation at different shear rates. Physiological Reports. 2 (7), 1-7 (2014).
- Zeng, Z., Fagnon, M., Nallan Chakravarthula, T., Alves, N. J. Fibrin clot formation under diverse clotting conditions: Comparing turbidimetry and thromboelastography. Thrombosis Research. 187, 48-55 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved