A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Blood Flow Imaging with Ultrafast Doppler
In This Article
Summary
This protocol shows how to apply ultrafast ultrasound Doppler imaging to quantify blood flows. After a 1 s long acquisition, the experimenter has access to a movie of the full field of view with axial velocity values for each pixel every ≈0.3 ms (depending on the ultrasound time of flight).
Abstract
The pulsed-Doppler effect is the main technique used in clinical echography to assess blood flow. Applied with conventional focused ultrasound Doppler modes, it has several limits. Firstly, a finely tuned signal filtering operation is needed to distinguish blood flows from surrounding moving tissues. Secondly, the operator must choose between localizing the blood flows or quantifying them. In the last two decades, ultrasound imaging has undergone a paradigm shift with the emergence of ultrafast ultrasound using unfocused waves. In addition to a hundredfold increase in framerate (up to 10000 Hz), this new technique also breaks the conventional quantification/localization trade-off, offering a complete blood flow mapping of the field of view and a simultaneous access to fine velocities measurements at the single-pixel level (down to 50 µm). This data continuity in both spatial and temporal dimensions strongly improves the tissue/blood filtering process, which results in an increase sensitivity to small blood flow velocities (down to 1 mm/s). In this method paper, we aim to introduce the concept of ultrafast Doppler as well as its main parameters. Firstly, we summarize the physical principles of unfocused wave imaging. Then, we present the Doppler signal processing main steps. Particularly, we explain the practical implementation of the critical tissue/blood flow separation algorithms and on the extraction of velocities from these filtered data. This theoretical description is supplemented by in vitro experiences. A tissue phantom embedding a canal with flowing blood-mimicking fluid is imaged with a research programmable ultrasound system. A blood flow image is obtained and the flow characteristics are displayed for several pixels in the canal. Finally, a review of in vivo applications is proposed, showing examples in several organs such as carotids, kidney, thyroid, brain and heart.
Introduction
Ultrasound imaging is one of the most commonly used imaging techniques in clinical practice and research activities. The combination of ultrasound wave emission in the biological tissues followed by the recording of the backscattered echoes allows the reconstruction of anatomical images, the so-called “B-Mode”. This method is perfectly adapted for soft tissue imaging, such as biological tissues, which typically permit the penetration of ultrasound over several centimeters, with a propagation speed of ≈1540 m/s. Depending on the center frequency of the ultrasound probe, images with a resolution from 30 µm to 1 mm are obtained. Furthermore, it is well known that the motion of an acoustic source, affects the physical characteristics of the associated waves. Particularly, the link between the frequency shifts of a wave relative to the speed of its source is described as the Doppler effect1, whose simplest manifestation is the changing siren’s pitch of a moving ambulance. Ultrasound imaging has long used this physical effect to observe the moving red blood cells2, and it proposes a variety of imaging modes commonly labelled “Doppler imaging”. These modes enable the assessment of blood flows in very different applications and organs, such as brain, heart, kidney or peripheral arteries.
Remarkably, most of the currently available ultrasound systems rely on the same technology, referred to as conventional ultrasound. The underlying principles are the following: an acoustic beam insonifies the field of view and is swept along the ultrasound transducer aperture. For each position of the beam, the echoes are recorded and converted into a line of the final image. By progressively moving the beam along the transducer, the whole field of view can be imaged line-per-line (Figure 1, left panel). This strategy was well adapted to the electrical constraints and computing power prevailing until the beginning of the 21st century. Nonetheless, it has several drawbacks. Among these, the final framerate is limited to a few hundred images per second by the beam scanning process. In terms of blood flow, this relatively low framerate affects the maximum flow velocities that can be detected, which is dictated by the sampling criteria of Shannon-Nyquist3. Moreover, conventional Doppler must deal with a complex tradeoff. In order to assess the blood flow velocity in a particular region of interest (ROI), several echoes coming from that ROI have to be successively recorded. This implies that the ultrasound beam is temporarily maintained in a fixed position. The longer the echo ensemble, the better the velocity estimation will be for that ROI. However, to produce a complete image of the field of view, the beam must scan the medium. Therefore, one can sense the conflict between these two constraints: holding the beam to precisely assess the velocity along one line, or moving the beam to produce an image. The different conventional Doppler modes (i.e., Color Doppler or Pulse Wave Doppler) directly reflect this tradeoff. Typically, the Color Doppler produces a low-fidelity flow map used for localizing the vessels4, and the Pulse Wave Doppler is then used to accurately quantify the flow in a previously identified vessel5.
These two limitations (low framerate and localization/quantification tradeoff) are overcome with very high-framerate emerging techniques. Among these, the synthetic aperture approach6 or the multiline transmit technique can be cited7. In this study, we focus on the so-called Ultrafast ultrasound method. Introduced two decades ago8,9,10, this method also relies on the emission/reception of ultrasounds, but with a radically different pattern. Indeed, instead of using a scanning focused beam, ultrafast imaging uses plane wave or diverging waves, which are able to insonify the field of view with a single emission. Following that single emission, the associated electronics is also able to receive and process the huge number of echoes originating from the whole field of view. At the end, an image can be reconstructed from a single emission/reception pattern11 (Figure 1, right panel). These unfocused emissions can have a low signal to noise ratio (SNR) due to the spread of the acoustical energy. This can be tackled by emitting several titled plane-waves (or diverging waves with different sources) and by adding the resulting images. This method is named “coherent compounding”12. Two major consequences arise. Firstly, the framerate only depends on the ultrasound time of flight and can reach typical values from 1 to 10 kHz. Secondly, this ensures the data continuity in both spatial and temporal dimensions, also referred to as spatiotemporal coherence. The conventional localization/quantification tradeoff is thus broken. This combination of a high framerate and spatiotemporal coherence has a tremendous impact on the ability to detect blood flows with ultrasound. As compared to conventional ultrasound, ultrafast ultrasound provides full characterization of the blood flow3. Practically, the user has access to the velocity time course in every pixel of the image, for the whole duration of the acquisition (typically ≈1 s), with a timescale given by the framerate (typically, a framerate of 5 kHz for a temporal resolution of 200 µs). This high framerate makes the method suitable for a wide range of application such as fast flow in moving organs like heart chambers13 or myocardium with the coronary micro-perfusion14. Furthermore, it has been shown that its spatiotemporal coherence strongly improves its ability to separate slow blood flow from background moving tissues, therefore increasing the sensitivity to micro-vascular flow15. This capacity gives access to the micro vasculature of the brain in both animals16 and humans17.
Hence, ultrafast ultrasound is well suited to image blood flow in a variety of situations. It is restricted to soft biological tissues and will be strongly affected by the presence of hard interfaces such as bones, or gas cavity such as the lung. The tuning of the physical parameters of the ultrasound sequence allows the study of both slow (down to 1 mm/s11,16) and fast flows (up to several m/s). A tradeoff exists between the spatial resolution and the depth of penetration. Typically, a resolution of 50 µm can be achieved at the cost of a penetration around 5 mm. Conversely, the penetration can be extended to 15-20 cm at the cost of a resolution of 1 mm. It is worth noting that most ultrafast scanners such as the one used in this article only provide 2D images.
Here, we propose a simple protocol to introduce the concept of Ultrafast Doppler imaging, using a programmable research ultrasound scanner and Doppler phantom mimicking a vessel (artery or vein) embedded in biological tissue.
Protocol
1. Doppler phantom preparation setup (Figure 2A)
- Connect the peristaltic pump, the blood mimicking fluid reservoir, the pulse dampener and the Doppler flow phantom with the plastic tubes.
- Choose the canal with a 4 mm diameter.
- Program the pump to eject 720 mL/min of fluid for 0.3 s and then to eject 50 mL/min for 0.7 s to respectively mimic the systole and diastole cardiac phases
- Run the pump and gently shake the pipes to expel potential air bubbles.
NOTE: The operator can choose a different canal diameter and different pump rate but will have to ensure that the ultrasound sequence is fast enough to acquire the fastest flow velocities. Eq. 3 presented later can help to design the sequence.
2. Ultrafast ultrasound scanner setup (Figure 2A)
- Connect the ultrafast-enabled research scanner to the host computer with the PCI express link.
- Change the transducer adapter on the ultrasound scanner to match the probe connector, then connect the probe.
- Run Matlab and activate the ultrasound scanner license.
NOTE: This section and the following implicitly assume the use of a Verasonics Vantage system.
3. Ultrasound sequence programming
- Using the examples scripts, design a conventional focused “B-Mode” (i.e., echography) sequence that will be used for probe positioning.
- Set the imaging depth to 50 mm.
- Set the focal depth to 35 mm.
- Using the examples scripts, design an ultrafast ultrasound sequence.
- Set the imaging depth to 50 mm.
- Program 3 tilted plane-waves at [-3,0,3] degree.
- Set the pulse repetition frequency (PRF) to 12 kHz.
- Use 4 half-cycles for the ultrasound waveform, with a center frequency depending on the probe used. A center frequency of 5.2 MHz is assumed here.
- Set the total duration to 1 s.
4. Probe positioning and data acquisition
- Apply ultrasound gel on the probe’s lens.
- Place the probe on the phantom and launch the B-Mode ultrasound sequence.
- Locate the canal of interest. The fluid appears darker than the surrounding tissue. Place the probe in longitudinal view.
- Manually maintain the probe in the position of interest.
- End the B-Mode sequence and launch the ultrafast sequence acquisition script.
5. Image reconstruction (Figure 2B)
- Once the sequence is over, save the raw data (also called Radio-Frequency data, “RF”).
- Launch the image reconstruction script using the ultrasound system default software. At the end of the process, the IQ data matrix should be created.
NOTE: The ultrasound echoes are recorded on each element of the probe and for each emission/reception, then stored in the RF data matrix. The image reconstruction applied the appropriate delay law to each channel and results in the so-called “IQ” (In-Phase/Quadrature) matrix. The complex IQ matrix has three dimensions: two for space (image depth and width) and one for time
6. Clutter filtering (Figure 2C)
NOTE: For steps 6-7, see the Matlab script provided in the Supplementary Material.
- Reshape the 3D (space x space x time) IQ matrix into a 2D (space x time) Casorati matrix, named IQr.
- Compute the singular value decomposition15 of IQr (Eq. 1).
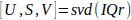 Eq. 1
Eq. 1 - Compute the Spatial Similarity Matrix C using the spatial singular vectors U as described by Baranger et al.18 (II, D), and identify the blood subspace boundaries N.
- Use this cutoff N to filter the IQ data as described in Demene et al.15 (II,C).
7. Flow visualization and velocity measurements (Figure 2C)
- Compute power Doppler map PD by integrating the envelope of the filtered data IQt along the temporal dimension (Eq. 2). The 3D coordinates z, x and t are respectively the depth, width and temporal dimension, nt and is the number of frames acquired.
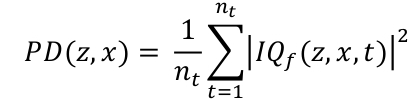 Eq. 2
Eq. 2 - Display the PD map in logarithm scale. To set the dynamic range, compute the mean PD in a region outside the canal and use this value in dB as the lower bound of the dynamic range. A typical dynamic range is [-30, 0] dB.
- Define a circular region of interest (ROI) on the image, containing 1 to 30 pixels.
- Average the IQf signal over the pixels of that ROI, to obtain a vector
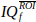 of nt time points.
of nt time points. - Compute and display the Doppler spectrogram of
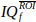 , using the square magnitude of the Short-Time Fourier Transform (STFT).
, using the square magnitude of the Short-Time Fourier Transform (STFT).
- Set the STFT window to a 60-samples Hann window.
- Set the STFT overlap to 90% of the window length.
- Overlay the center frequency at each time point of the spectrogram.
- Convert the frequency f values into blood axial velocities vz using the Doppler formula (Eq. 3). c0 is the speed of sound in the medium and fTW the center frequency of the transmitted ultrasound waveform (here 5.2 MHz).
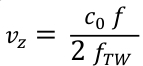 Eq. 3
Eq. 3
Results
The quality of the acquisition and the post-processing is firstly assessed by visual inspection. The shape of the canal must be clearly visible in the power Doppler image, and the tissue area must appear dark. If the power Doppler signal is not restricted to the canal, it can mean that either the clutter filter step went wrong (SVD threshold is too low), or the probe experienced a strong movement during the acquisition.
After visual inspection, the study of the spectrogram inside the canal can...
Discussion
Several variations are possible around the main frame of this protocol.
Hardware concerns
If the user supplies its custom host computer, the motherboard and the computer’s case must have an available PCI express slot. The CPU must also have enough PCIe lanes to handle all the devices.
Probe selection
The ultrasound probe (also named transducer) is chosen according to the spatial resolution needed and to the geometr...
Disclosures
No conflict of interest
Acknowledgements
We would like to thank Shreya Shah for her proofreading and advice.
Materials
| Name | Company | Catalog Number | Comments |
| Blood-mimicking fluid | CIRS Inc, Norfolk, Virginia, USA | 069DTF | |
| Doppler flow phantom | CIRS Inc, Norfolk, Virginia, USA | ATS523A | |
| Matlab | MathWorks, Natick, Massachusetts, United States | ||
| Peristaltic pump / Doppler flow pump | CIRS Inc, Norfolk, Virginia, USA | 769 | Include tubings and pulse dampener |
| Transducer adpter | Verasonics, Kirkland, Washington, USA | UTA 408-GE | |
| Ultrafast ultrasound research scanner | Verasonics, Kirkland, Washington, USA | Vantage 256 | |
| Ultrasound probe/transducer | GE Healthcare | GE 9L-D |
References
- Doppler, C. . Ueber das farbige Licht der Doppelsterne und einiger anderer Gestirne des Himmels. , (2020).
- Bonnefous, O., Pesqué, P. Time domain formulation of pulse-Doppler ultrasound and blood velocity estimation by cross correlation. Ultrasonic Imaging. 8 (2), 73-85 (2004).
- Bercoff, J., et al. Ultrafast compound doppler imaging: Providing full blood flow characterization. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 58 (1), 134-147 (2011).
- Evans, D. H., Jensen, J. A., Nielsen, M. B. Ultrasonic colour Doppler imaging. Interface Focus. 1 (4), 490-502 (2011).
- Nuffer, Z., Rupasov, A., Bekal, N., Murtha, J., Bhatt, S. Spectral Doppler ultrasound of peripheral arteries: a pictorial review. Clinical Imaging. 46, 91-97 (2017).
- Jensen, J. A., Nikolov, S. I., Gammelmark, K. L., Pedersen, M. H. Synthetic aperture ultrasound imaging. Ultrasonics. 44, (2006).
- Tong, L., Ramalli, A., Jasaityte, R., Tortoli, P., D'Hooge, J. Multi-transmit beam forming for fast cardiac imaging-experimental validation and in vivo application. IEEE Transactions on Medical Imaging. 33 (6), 1205-1219 (2014).
- Tanter, M., Bercoff, J., Sandrin, L., Fink, M. Ultrafast compound imaging for 2-D motion vector estimation: application to transient elastography. IEEE Transactions on Ultrasonics, Ferroelectrics and Frequency Control. 49 (10), 1363-1374 (2002).
- Udesen, J., et al. High frame-rate blood vector velocity imaging using plane waves: Simulations and preliminary experiments. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 55 (8), 1729-1743 (2008).
- Hansen, K. L., Udesen, J., Gran, F., Jensen, J. A., Bachmann Nielsen, M. In-vivo examples of flow patterns with the fast vector velocity ultrasound method. Ultraschall in der Medizin. 30 (5), 471-477 (2009).
- Tanter, M., Fink, M. Ultrafast imaging in biomedical ultrasound. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 61 (1), 102-119 (2014).
- Montaldo, G., Tanter, M., Bercoff, J., Benech, N., Fink, M. Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography. IEEE Transactions on Ultrasonics, Ferroelectrics and Frequency Control. 56 (3), 489-506 (2009).
- Papadacci, C., Pernot, M., Couade, M., Fink, M., Tanter, M. High-contrast ultrafast imaging of the heart. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 61 (2), 288-301 (2014).
- Maresca, D., et al. Noninvasive Imaging of the Coronary Vasculature Using Ultrafast Ultrasound. JACC: Cardiovascular Imaging. 11 (6), 798-808 (2018).
- Demené, C., et al. Spatiotemporal Clutter Filtering of Ultrafast Ultrasound Data Highly Increases Doppler and fUltrasound Sensitivity. IEEE Transactions on Medical Imaging. 34 (11), 2271-2285 (2015).
- Demené, C., et al. 4D microvascular imaging based on ultrafast Doppler tomography. NeuroImage. 127, 472-483 (2016).
- Demené, C., et al. Ultrafast Doppler reveals the mapping of cerebral vascular resistivity in neonates. Journal of Cerebral Blood Flow and Metabolism. 34 (6), 1009-1017 (2014).
- Baranger, J., Arnal, B., Perren, F., Baud, O., Tanter, M., Demene, C. Adaptive Spatiotemporal SVD Clutter Filtering for Ultrafast Doppler Imaging Using Similarity of Spatial Singular Vectors. IEEE Transactions on Medical Imaging. 37 (7), 1574-1586 (2018).
- Demené, C., et al. Ultrafast Doppler Reveals the Mapping of Cerebral Vascular Resistivity in Neonates. Journal of Cerebral Blood Flow & Metabolism. 34 (6), 1009-1017 (2014).
- Goudot, G., et al. Wall Shear Stress Measurement by Ultrafast Vector Flow Imaging for Carotid Stenosis. Ultraschall in der Medizin - European Journal of Ultrasound. , (2019).
- Demené, C., Mairesse, J., Baranger, J., Tanter, M., Baud, O. Ultrafast Doppler for neonatal brain imaging. NeuroImage. 185, 851-856 (2019).
- Villemain, O., et al. Ultrafast Ultrasound Imaging in Pediatric and Adult Cardiology. JACC: Cardiovascular Imaging. , (2019).
- Provost, J., Papadacci, C., Demene, C., Gennisson, J. L., Tanter, M., Pernot, M. 3-D ultrafast doppler imaging applied to the noninvasive mapping of blood vessels in Vivo. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 62 (8), 1467-1472 (2015).
- Osmanski, B. F., Montaldo, G., Fink, M., Tanter, M. In vivo out-of-plane Doppler imaging based on ultrafast plane wave imaging. IEEE International Ultrasonics Symposium, IUS. 62 (4), 76-79 (2013).
- Kim, M. W., Zhu, Y., Hedhli, J., Dobrucki, L. W., Insana, M. F. Multi-dimensional Clutter Filter Optimization for Ultrasonic Perfusion Imaging. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 65 (11), 2020-2029 (2018).
- Chau, G., Li, Y. L., Jakovljevic, M., Dahl, J., Rodr, P. . Wall Clutter Removal in Doppler Ultrasound using Principal Component Pursuit. , (2018).
- Tierney, J., Baker, J., Brown, D., Wilkes, D., Byram, B. Independent Component-Based Spatiotemporal Clutter Filtering for Slow Flow Ultrasound. IEEE Transactions on Medical Imaging. , 1-1 (2019).
- Zhang, N., Rivaz, H. Clutter Suppression in Ultrasound: Performance Evaluation and Review of Low-Rank and Sparse Matrix Decomposition Methods. BioMedical Engineering Online. 19, 37 (2020).
- Guidi, G., Licciardello, C., Falteri, S. Intrinsic spectral broadening (ISB) in ultrasound Doppler as a combination of transit time and local geometrical broadening. Ultrasound in Medicine and Biology. 26 (5), 853-862 (2000).
- Cloutier, G., Shung, K. K., Durand, L. G. Experimental Evaluation of Intrinsic and Nonstationary Ultrasonic Doppler Spectral Broadening in Steady and Pulsatile Flow Loop Models. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 40 (6), 786-795 (1993).
- Winkler, A. J., Wu, J. Correction of intrinsic spectral broadening errors in doppler peak velocity measurements made with phased sector and linear array transducers. Ultrasound in Medicine and Biology. 21 (8), 1029-1035 (1995).
- Osmanski, B. F., Bercoff, J., Montaldo, G., Loupas, T., Fink, M., Tanter, M. Cancellation of Doppler intrinsic spectral broadening using ultrafast Doppler imaging. IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control. 61 (8), 1396-1408 (2014).
- Sauvage, J., et al. A large aperture row column addressed probe for in vivo 4D ultrafast doppler ultrasound imaging. Physics in Medicine and Biology. 63 (21), (2018).
- Correia, M., Provost, J., Tanter, M., Pernot, M. 4D ultrafast ultrasound flow imaging: in vivo quantification of arterial volumetric flow rate in a single heartbeat. Physics in Medicine and Biology. 61 (23), 48-61 (2016).
- Center for Devices and Radiological Health. FDA Information for Manufacturers Seeking Marketing Clearance of Diagnostic Ultrasound Systems and Transducers. Center for Devices and Radiological Health. , (2008).
- I, IEC 62127-1 - Measurement and characterization of medical ultrasonic fields up to 40 MHz. IEC. , 61010-61011 (2013).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved