Method Article
Rapid In Vivo Fixation and Isolation of Translational Complexes from Eukaryotic Cells
* These authors contributed equally
In This Article
Summary
We present a technique to rapidly stabilize translational (protein biosynthesis) complexes with formaldehyde crosslinking in live yeast and mammalian cells. The approach enables dissecting transient intermediates and dynamic RNA:protein interactions. The crosslinked complexes can be used in multiple downstream applications such as in deep sequencing-based profiling methods, microscopy, and mass-spectrometry.
Abstract
Rapid responses involving fast redistribution of messenger(m)RNA and alterations of mRNA translation are pertinent to ongoing homeostatic adjustments of the cells. These adjustments are critical to eukaryotic cell survivability and 'damage control' during fluctuating nutrient and salinity levels, temperature, and various chemical and radiation stresses. Due to the highly dynamic nature of the RNA-level responses, and the instability of many of the RNA:RNA and RNA:protein intermediates, obtaining a meaningful snapshot of the cytoplasmic RNA state is only possible with a limited number of methods. Transcriptome-wide, RNA-seq-based ribosome profiling-type experiments are among the most informative sources of data for the control of translation. However, absence of a uniform RNA and RNA:protein intermediate stabilization can lead to different biases, particularly in the fast-paced cellular response pathways. In this article, we provide a detailed protocol of rapid fixation applicable to eukaryotic cells of different permeability, to aid in RNA and RNA:protein intermediate stabilization. We further provide examples of isolation of the stabilized RNA:protein complexes based on their co-sedimentation with ribosomal and poly(ribo)somal fractions. The separated stabilized material can be subsequently used as part of ribosome profiling-type experiments, such as in Translation Complex Profile sequencing (TCP-seq) approach and its derivatives. Versatility of the TCP-seq-style methods has now been demonstrated by the applications in a variety of organisms and cell types. The stabilized complexes can also be additionally affinity-purified and imaged using electron microscopy, separated into different poly(ribo)somal fractions and subjected to RNA sequencing, owing to the ease of the crosslink reversal. Therefore, methods based on snap-chilling and formaldehyde fixation, followed by the sedimentation-based or other type of RNA:protein complex enrichment, can be of particular interest in investigating finer details of rapid RNA:protein complex dynamics in live cells.
Introduction
Living organisms are subject to dynamic intra- and extracellular changes across their lifespans, which require rapid responses to maintain homeostasis and ensure survival. To allow environmental adaptation, eukaryotic cells adjust their metabolism via gene expression control. Gene expression control can be exerted during transcription and/or translation; with translational responses generally occurring more rapidly1,2,3,4. For example, translational changes typically arise within 1-30 min of the stress onset, while transcription-level alterations follow hours after stress exposure3,4,5. Alterations to translation output are achieved more rapidly due to the persistent availability of messenger (m)RNA molecules in the cytoplasm. Conversely, at the transcription level, new mRNA molecules must be synthesized, and in eukaryotes, processed and exported from the nucleus, producing extensive delays in the response time2,4,6,7,8.
Acute translational response to stress is generally characterized by an overall decrease in translation output, with the selective upregulation of proteins necessary for cell survival1,3,4,9. Decreasing the protein production output is thought to be crucial due to the high energy expense of the process3,7. To facilitate the selective inhibition and upregulation, translational responses are served by a range of complex regulatory mechanisms. Regulation can be exerted across all phases of translation: initiation, elongation, termination of polypeptide biosynthesis and ribosomal recycling10,11,12,13, but is exhibited most strongly at the initiation phase5,7,9,10,13. During initiation, the small ribosomal subunit (SSU), assisted by eukaryotic initiation factors (eIFs), binds to, and scans the 5' untranslated region (UTR) of mRNA until a start codon is recognized2,5,6,8,11,12,13. Regulatory mechanisms often target eIFs affecting attachment, scanning, and start codon recognition. For example, the initiation factor eIF2, an essential translation factor that aids in the recruitment of an initiator Met-tRNAiMet to the SSU, is often targeted in eukaryotes under stress conditions4,6,11. In yeast, phosphorylation of this factor can be induced under nutrient deprivation and osmotic stress1,4,11,14,15, and in mammalian cells, amino acid starvation, endoplasmic reticulum (ER) stress, UV stress, viral infection, and altered oxygen levels may trigger this response8,9,11. Rapid upregulation of specific mRNA translation is evident in the mammalian cell response to hypoxia, which exhibits a global rapid translation inhibition and selective upregulation of hypoxia-inducible factors (HIFs) biosynthesis. HIFs are transcription factors, which then elicit longer term cellular reprogramming at the DNA transcription level8,9,16. Similar responses have been observed in yeast under heat stress, with rapid translational expression of Heat Shock Proteins (HSPs) followed by delayed transcription-level responses17,18. In addition to nutrient deprivation and heat shock, translational responses in yeast have been studied under varying oxygen8,19, salinity5, phosphate, sulfur20,21 and nitrogen22,23 levels. This research has widespread implications for the industrial uses of yeast, such as baking and fermentation24,25. Translational responses may also be instrumental in furthering understanding of diseases such as neurodegenerative disorders and heart disease, that are characterized by intracellular stresses like oxidative stress. Overall, translational responses are integral to the gene expression control and facilitate rapid adaptation to a broad range of stress conditions in eukaryotic organisms.
In order to study translational responses, methods are required that provide minimally distorted snapshots of the translation landscape. Polysome profiling is a classical approach used in the study of translation across mRNA, involving the separation of poly(ribo)somal fractions of mRNA via ultracentrifugation through sucrose gradients26,27. The approach may be used to explore levels of translation for individual mRNAs (with the detection methods such as reverse transcription and polymerase chain reaction, RT-PCR26), or globally in conjunction with high-throughput techniques (microarray or RNA-seq28,29). A more evolved approach is ribosome profiling, that allows the study of positions of elongating ribosomes along an mRNA molecule at a genome-wide scale, as well as the inference of efficiency of translation across transcriptome and utilization of the main and alternative start sites30,31. Ribosome profiling involves the isolation and sequencing of mRNA fragments protected by ribosomal presence over them. Ribosome profiling has provided considerable insight into translation dynamics across a number of conditions, including hypoxic stress, heat shock and oxidative stress31,32. The technique has been adapted to multiple source material types, including yeast and mammalian cells.
While polysome and ribosome profiling have been fundamental in extending the capabilities of research in translation, the process of translation includes various translational intermediates and complexes that are difficult to capture with these methods11,13. An additional limitation stems from the lack of ability to study rapid response types, as translational complexes are either stabilized in vivo by the addition of specific translation inhibitors (antibiotics), leading to certain ribosome distribution artifacts, or ex vivo upon cell lysis specifically (antibiotics) or unspecifically (high salt or magnesium ions), leading to the deprivation of the shorter-lived or less stable intermediates33,34,35.
Formaldehyde is widely used to crosslink nucleic acids and proteins, such as in chromatin immunoprecipitation (ChIP) and crosslinking immunoprecipitation (CLIP) studies. Its small size and excellent cell permeability allow for a rapid in vivo action36. Based on the rapid formaldehyde crosslinking, the ribosome profiling approach has been extended with the Translation Complex Profile Sequencing (TCP-seq)10,36,37,38,39,40. TCP-seq, first developed in yeast, allows the capture of all translation intermediates, including scanning or post-termination SSU complexes and multiple ribosomal configurations37,38,41,42. The method has been utilized in several studies10,38,39,41,42, some of which use a combinatory approach of both translation inhibitors and formaldehyde crosslinking to facilitate the arrest of translation. A further modified version of the technique, selective TCP-seq39, has recently been employed to include immunopurification of the crosslinked complexes, broadening the scope of the TCP-seq applications. The rapid, efficient and reversible nature of formaldehyde crosslinking makes these approaches suitable for studying transient mRNA:translation complex interactions, particularly in the context of highly dynamic translation-level response pathways.
Here we detail the processes of in vivo formaldehyde crosslinking for the purpose of comprehensive translation complex stabilization and isolation. We provide separate protocols nuanced for yeast and mammalian cells (Figure 1). We further outline examples of the subsequent use of the crosslink-stabilized material (Figure 1), such as for co-purified protein factor detection using immunoblotting (western-blotting), immuno-assisted purification (or 'immunoprecipitation'; IP) and enrichment of translational complexes containing specific factors of interest, electron microscopy and RNA sequencing.
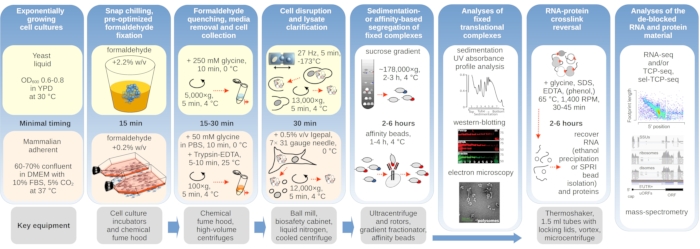
Figure 1: Schematic depicting an overview of the typical experimental setup. Main steps of in vivo formaldehyde stabilization of translational complexes are depicted as a flowchart, supplemented by information about the key necessary instruments. Potential downstream applications of the crosslinked material are outlined, including examples which have been successfully employed but not directly covered in this protocol, such as SPRI bead purification of RNA, RNA sequencing, and mass-spectrometry. Please click here to view a larger version of this figure.
Protocol
1. Yeast cell protocol
- Yeast cell culture and fixation
NOTE: Cell fixation and harvesting are adapted from10,38 with modifications.- Set up 1 L yeast cell culture (wild-type (WT) BY4741 are given as an example) in an orbital shaker with the starting optical density of no more than 0.05 AU at 600 nm (OD600) in suitable media (1% w/v of yeast extract, 2% w/v of peptone, 2% w/v of dextrose (glucose), 40 mg/L of adenine sulfate (YPD) used as an example) under the desired conditions (30 °C used in this experiment).
- Set up a preparative centrifuge with compatible rotor and centrifuge bottles for pelleting the liquid suspension culture of yeast cells. For glucose starvation experiments, pellet the cells once the optical density of 0.6-0.8 AU at 600 nm (OD600) is reached, using a brief centrifugation at 30 °C, 5,000 x g for 1 min.
NOTE: Keep record of the OD of the growing cells and let the cells grow until the OD600 reaches 0.6-0.8 AU, if the exponential growth phase is of an interest. - Resuspend the pellet immediately in warm (30 °C) YP media containing no or low (0.25% w/v) added glucose and incubate the culture for a further 10 min at 30 °C in an orbital shaker-incubator.
NOTE: Media composition might affect subsequent crosslinking efficiency. This protocol was tested using YPD only. When performing starvation experiments, adhering to the timing and minimizing the delays between procedures is critical. - Once the cells are ready, set up an ice box inside the fume hood with a beaker containing 250 g of clean crushed water ice. Ensure 25 mL stripettes and freshly purchased methanol-stabilized 37% w/v formaldehyde solution are accessible inside the hood. Pour the 1 L culture into the beaker containing 25% w/v of crushed water ice.
NOTE: Keep the cells on ice throughout all subsequent operations until the cells are frozen, unless indicated otherwise. - Add 75 mL of 37% w/v of formaldehyde solution to a final concentration of 2.2% w/v and intensely stir the mixture until the ice melts.
- Once the ice is melted, set up a timer for 10 min.
NOTE: Adhere to the recommended timings and temperature regimen to attain reproducible fixation results. - After incubating for 10 min, transfer the culture into the precooled centrifuge bottles and pellet the cells by centrifugation at 4 °C, 5,000 x g for 5 min. While this spin is on, precool a 50 mL tube and keep freshly prepared buffer A (containing glycine to neutralize any remaining formaldehyde) on ice.
NOTE: Refer to the supplied table for the exact buffer compositions. - After centrifuging, place the centrifuge tubes onto ice with the pellet side in contact with the ice. Bring the tubes into the fume hood and discard the supernatant into a formaldehyde waste container.
- Resuspend the cell pellet from all tubes in 20 mL of buffer A using a 25 mL stripette and transferring to a 50 mL tube.
NOTE: This wash is critical for avoiding irreproducible crosslinking and the buffer addition must not exceed 20 min of time from the cells' harvest. - Make the volume up to 40 mL with buffer A and collect the washed cells by centrifugation at 4 °C, 5,000 x g for 5 min.
- Discard the supernatant and resuspend the cell pellet in 40 mL of buffer A1, which is buffer A not containing glycine, to remove any glycine contamination.
- Pellet cells again by centrifugation at 4 °C, 5,000 x g for 5 min.
- Repeat the washes with buffer A1 one more time. Discard the supernatant and place the cell pellet on ice. Weigh the tube with the pellet (wet cell mass should be ~1 g per 1 L of the cell culture).
- Yeast cell disruption and cytosol collection
- Fill a polystyrene foam box lined with aluminum foil with liquid nitrogen to a depth of approximately 3 cm. Place a 50 mL tube upright in the box.
- Resuspend the pellet (~1 g wet cell mass) in 550 µL of buffer A2 by pipetting and vortexing for 10 s. Add 10 µL of 40 U/µL of RNase inhibitor and vortex again for 10 s.
CAUTION: Wear appropriate protective equipment, such as thermally insulated gloves, when handling liquid nitrogen. Ensure that any container used to hold liquid nitrogen does not leak, and that the tube rack inside will not float up or fall on its side. Work in a well-ventilated area to avoid oxygen depletion. - Using a 1 mL pipette, drip the cell suspension into the 50 mL tube containing the liquid nitrogen.
NOTE: The dripping must be performed slowly and carefully to avoid aggregation of the droplets. Ensure the droplets freeze before introducing new droplets. - Transfer the 50 mL tube with the frozen cell suspension droplets to room temperature and wait until the liquid nitrogen evaporates completely. Seal the tube with its cap and store the cell pellets at -80 °C or immediately proceed further.
CAUTION: Ensure that the liquid nitrogen is completely evaporated before sealing the tube. Leftover liquid nitrogen in a sealed tube can cause a hazardous pressure build-up. - To prepare for the next step, precool 1.5 mL nuclease-free tubes and 10 mL stainless steel grinding jars on dry ice.
- Transfer the frozen cell suspension droplets into the jars using a clean, sterile spatula.
CAUTION: Ensure that the grinding jars are tightly sealed. - Submerge the grinding jars into the liquid nitrogen for 1 min ensuring the liquid phase remains below the junction. Set up a cryo mixer mill at 27 Hz for agitation for 1 min.
NOTE: Always balance the grinding canister with another one of the same model even if the sample requires only one canister for processing. - Agitate the sealed grinding jars at 27 Hz for 1 min in the mixer mill.
- Re-cool the grinding jars in liquid nitrogen as before and shake at 27 Hz for 1 min further in the mixer mill.
- Transfer the jars to the ice box containing dry ice along with the 1.5 mL nuclease-free tubes. Using a small steel spatula, transfer the resultant powdered sample into the tubes in ~100 mg aliquots, and store the tubes at -80 °C.
NOTE: It is recommended to use ~600 mg of the sample per experiment comprising polysome sedimentation profile analysis, separation of the cytosol into translated and non-translated fractions, and further separation of the translated fraction into SSU, ribosome, and disome fractions upon RNase digestion.
- Separation of the fixed (poly)ribosomal complexes from the non-translated fractions of the cytosol
NOTE: The procedure established earlier10,38 is generally followed to enrich translated RNA based on its co-sedimentation with (poly)ribosomes. A more refined approach to separating the translated and non-translated cytosol fractions is introduced here, eliminating the need to precipitate and subsequently re-solubilize the material.- Prepare 2.5 mL linear 10%-20% w/v sucrose gradients with buffer B using the freeze-thaw method43 in thin wall ultracentrifuge tubes (5 mL, 13 x 51 mm).
NOTE: The freeze-thaw method is performed by the sequential addition and freezing of buffered sucrose layers with linearly regressing concentrations on top of each other. See Supplementary Table 1 for details. - To create a discontinuous 50% w/v sucrose cushion, upon the linear gradients thawing and stabilizing, slowly dispense 0.5 mL of 50% sucrose in buffer B directly onto the bottom of the tubes using a 1 mL syringe attached to 19 G x 1.5" needle or a glass capillary of similar/suitable dimensions. Before dispensing, carefully and slowly drive the tip of the needle or capillary from the top to bottom of the pre-formed sucrose gradients, avoiding any disturbance, until it reaches the tube bottom.
NOTE: See Supplementary Table 1 for instructions on preparation of buffer B. - Carefully balance the gradients by removing the top portions or layering more 10 w/v of sucrose in buffer B and keep them ice-cold or at 4 °C.
NOTE: The discontinuous gradient with the bottom 50% sucrose layer is needed to collect material with higher sedimentation rate without precipitating it on the tube wall. - Thaw ~100 mg of the frozen cell powdered sample at room temperature and immediately place on ice. Mix in 150 µL of buffer A2 by pipetting, add RNase inhibitor to 1 U/µL and mix by vortexing (avoid excessive foaming and mixing with the gaseous phase) for 10 s.
NOTE: Continue all operations while keeping the material on ice, unless otherwise indicated. - Pellet the cell debris by centrifuging the tubes at 4 °C, 13,000 x g for 5 min and recover the clarified supernatant (~150 µL) in a new 1.5 mL low protein binding tube.
- Load the resultant clarified mixture onto the discontinuous sucrose gradient tubes from step 1.3.3 and carefully balance them.
- Ultracentrifuge the tubes in a medium volume swing-bucket rotor at 4 °C, with average g-force 287,980 x g (k-factor 49) for 1 h 30 min.
NOTE: These conditions have been pre-optimized (using post-ultracentrifugation gradient UV absorbance trace analysis) to retain the free (non-(poly)ribosomal) SSUs and LSUs (large ribosomal subunit) in the top (10%-20% sucrose) portion of the gradient while concentrating the (poly)ribosomal fraction in the bottom (50%) sucrose cushion without pelleting the material. - Use a new sterile 1 mL syringe equipped with a 19 G x 1.5" needle to collect the translated cytosol fraction. Place the 5 mL gradient on a stable rack ensuring the bottom of the tube is visible.
- From the top of the tube, stick the needle straight into the bottom of the gradient (without puncturing the tube) and gently, without creating any bubbles, draw exactly 0.5 mL of the bottom solution containing the translated RNA pool.
NOTE: Ensure this step is performed in a cold room and the tube is held firmly. It is recommended to draw the entire 0.5 mL in a single upstroke motion to avoid disturbance of the gradient. - Confirm the (poly)ribosomal presence and the depletion of the SSU, LSU and lighter fractions in the resultant mixture by absorbance readout of the sucrose gradient upon ultracentrifugation run.
- Concentrate the collected translated RNA pool from the previous step to 100 µL using ultrafiltration in a micro-concentration device with 10 kDa cut-off regenerated cellulose membrane.
NOTE: Pre-wash the micro-concentration device's membrane with 0.5 mL of buffer 1 (see Figure 2a) and use spin conditions (g) recommended by the manufacturer. - Further dilute the material from the previous step five times (add 400 µL) with buffer 1 and concentrate back to 200 µL, to allow for a smaller volume as well as partial removal of the sucrose.
NOTE: It is recommended to store the resultant mixtures at -80 °C for up to 6 months and use as an input material for the 'total translated RNA' RNA-seq library construction, or the RNase digestion step of the TCP-seq library construction. The 'non-translated' cytosol fraction can be recovered from the top of the gradient using a similar procedure and stored at -80 °C.
- Prepare 2.5 mL linear 10%-20% w/v sucrose gradients with buffer B using the freeze-thaw method43 in thin wall ultracentrifuge tubes (5 mL, 13 x 51 mm).
- RNase digestion of the fixed (poly)ribosomal complexes and separation of digested material into small ribosomal subunit (SSU), monoribosomal (ribosomes, RS), and diribosomal (disomes, DS) fractions
NOTE: The procedure generally follows an approach described previously10,38 but a modified gradient type, separation time, acceleration and RNase digestion conditions are employed, to achieve best resolution across all three isolated fractions.- Prepare carefully balanced 12.5 mL linear 10%-40% w/v sucrose gradients made with buffer 1 in 13 mL thin wall polypropylene tubes, 14 x 89 mm, using the freeze-thaw method43 as described in step 1.3.1 and note therein.
- Thaw at room temperature and immediately transfer the samples on ice or take the concentrated and sucrose-depleted translated cytosol fraction from step 1.3.12.
NOTE: Continue all procedures on ice unless otherwise indicated. - Digest the translated cytosol fraction by mixing in 4.5 U of E. coli RNase I per 1 OD260 unit of the fraction for 30 min at 23 °C. Immediately add and mix in by pipetting the RNase inhibitor capable of inactivating RNase I to 0.25 U/µL to the mixture, to inactivate RNase I.
NOTE: Use RNase inhibitor capable of inhibiting RNase I. Derive AU260 by using AU260 = (Absorbance at 260 nm standardized to optical density units equivalent to 1 cm optical path x volume of the lysate in µL) / 1,000. - Immediately transfer the samples to ice.
CAUTION: It is critical to adhere to the recommended conditions of digestion and carefully measure the amount of the added RNase I. The RNase I unit referred to here is defined as the amount of the enzyme required to produce 1 µg of acid-soluble material from mouse liver RNA in 30 min at 37 °C. RNase I batches may have undocumented variations in activity and may require experimentation to achieve optimal digestion conditions. If the enzyme stock is too concentrated, it is recommended to dilute it with buffer 1 to avoid pipetting very small volumes of the solution. - Load the reaction mixtures onto the 10%-40% w/v sucrose gradients from step 1.4.1.
NOTE: Use final volumes in the range of 150-300 µL per gradient. Each purification requires minimally two gradients. Use different input volumes of the material (lower AU260, 10-11 AU260, for DS and comparatively higher AU260, 13-14 AU260, for SSU or RS) to achieve optimal separation. - Ultracentrifuge the tubes in a medium volume swing-bucket rotor at 4 °C with average g-force 178,305 x g (k-factor 143.9) for 3 h 30 min.
CAUTION: If spare balance tubes are needed, equalize their mass and mass distribution with the sample-containing tubes. Use spare sucrose gradients overlaid with an amount of buffer equivalent to that of the sample overlay and not tubes with uniform sucrose concentration. - Set up a gradient fractionator device at least 30 min before the ultracentrifugation spin completion, including filling in the 0.2 µm filtered heavy chase solution (e.g., 60% sucrose in deionized water as used here) into the displacement pump.
NOTE: It is recommended to de-contaminate the lines and tubings of the fractionator using deionized water, followed by 1%-2% SDS solution in deionized water, deionized water, and finally 80% ethanol in deionized water solution before and after the runs. - Adjust the absorbance readout baseline by first filling the system with deionized water and zeroing the optics as per the manufacturer's recommendations, and then compensating the baseline shift using a spare unloaded 14 x 89 mm sucrose gradient made with a buffer identical to the sample tubes (e.g., buffer 1).
NOTE: Use the same displacement speed to make the adjustments as for the sample readout, such as 1.5 mL/min. - Measure the displacement system dead volume by accurately counting time between the solution first entering the optical path of the detector and first appearing at the fraction collector output.
NOTE: With the recommended speed of 1.5 mL/min, the fractionation can be performed at room temperature. It is recommended to immediately transfer the collected fractions on ice. - Perform fractionation using live absorbance readout at 254 nm, 1.5 mL/min displacement speed, and in-line fraction detection based on the expected sedimentation position and absorbance profile of the samples. Use collector tube switching with a time delay corresponding to the dead volume as measured before.
- Isolate fractions corresponding to the positions and mobility of the SSU, RS, and DS complexes and collect them into new low protein binding 1.5 mL microcentrifuge tubes; immediately transfer the isolated fractions on ice and freeze if not processed further right away.
NOTE: It is recommended to immediately flash-freeze the collected fractions in dry ice or liquid nitrogen and store at -80 °C or below for up to 6 months.
- De-crosslinking of the ribosomal complexes and isolation of the RNA to construct RNA-seq libraries
- To de-block/reverse the crosslinks and isolate the RNA away from the associated proteins, transfer approximately half of the entire sucrose gradient fractions into new low nucleic acid binding nuclease-free polypropylene 1.5 mL microcentrifuge tubes (350 µL per tube) with lid safety/locking devices.
- Supplement the mixtures with 40 µL of 100% stop solution (10% SDS w/v and 100 mM EDTA), 4 µL of 1 M Tris-HCl pH 2 at 25 °C (to 10 mM), 1.6 µL of 2.5 M glycine (to 10 mM) and deionized nuclease-free water to obtain the final volume of 400 µL.
- Mix the contents of the tubes by pipetting and transfer the tubes at room temperature.
- Add equal volume of the acidic phenol:chloroform:isoamyl alcohol 125:24:1 (pH 4.0-5.0) mixture to each tube. Vigorously shake the mixtures for 2 min using a vortex mixer set to maximum speed.
CAUTION: Phenol and chloroform are corrosive and toxic. Avoid physical contact with the liquids and work in a well-ventilated area or under a fume hood. Always use gloves, lab coat and protective goggles or a face shield when working with phenol or chloroform. - Place the tubes in a thermoshaker and continuously shake at 65 °C, 1,400 rpm for 30 min.
- Facilitate phase aggregation by centrifuging the mixture at 12,000 x g for 10 min at room temperature.
- Collect the upper aqueous phases and transfer them into fresh low nucleic acid binding 1.5 mL tubes.
NOTE: To avoid cross-contamination, do not attempt to recover the aqueous phases completely. A reasonable recovery volume is 300-350 µL. - Supplement the collected aqueous phases with 0.1 volumes of 3 M sodium acetate (pH 5 at 25 °C), 20 µg of glycogen (using 5 µg/µL stock) and 2.5 volumes of absolute ethanol. Carefully mix the solutions by vortexing the tubes for 1 min.
- Precipitate the RNA by incubating the samples at -20 °C for at least 2 h (recommended overnight).
- Warm the tubes to room temperature and mix by vortexing.
NOTE: Pre-warming of the tubes and subsequent centrifugation at room temperature (without forced chilling) help to reduce salt and phenol co-precipitation and carryover. These conditions should not result in material loss or inefficiency of RNA collection if performed as described and using sufficiently pure ethanol. - Pellet the RNA precipitate by centrifuging the tubes at 12,000 x g for 30 min at room temperature.
- Discard the supernatant and wash the pellet twice with 80% v/v ethanol, collecting it each time by centrifugation at 12,000 x g for 10 min at room temperature.
- Dry the RNA pellets by opening the tube lids and placing the opened tubes in a dry-block heater set to 45 °C for 10 min. Dissolve the resultant dried pellet in 20 µL of 1x HE buffer.
- Estimate the resultant RNA concentration using UV absorbance spectrum measurement.
NOTE: RNA fragment length and total amount can be further assessed using denaturing gel-electrophoresis, such as in an automated fluorescence-based capillary gel electrophoresis apparatus.
- Selective co-immunopurification of the SSUs by the tagged eIFs and western blot analysis of the selective SSU enrichment
NOTE: Use ~15 AU (260 nm) of the digested and sedimentation-segregated SSU fraction from step 1.4.11 to perform affinity purification using magnetic IgG beads. Save ~5% of the SSU fraction as input control (Input fraction, I). eIF4A-tagged (TIF1-TAP; Tandem Affinity Purification tag) yeast strain was used which also makes it possible to detect eIF4A by probing for the TAP-tag using anti-TAP antibody.- Transfer 100 µL of magnetic IgG beads suspension (1 mg of the beads were used for each 15 AU (260 nm) of the lysate or fraction) into a new low protein binding 1.5 mL tube; collect the beads using magnetic rack and aspirate them.
- Wash the magnetic beads twice with 1 mL of buffer 1 by using sequential resuspension by pipetting and collection using the magnetic rack.
- After washing, collect and decant the beads, while keeping them on the magnetic rack.
- Add the SSU fraction to the washed beads and incubate the mixture for 4 h with rotation at 4 °C in a cyclomixer set at ~20 rpm.
- Collect the beads using the magnetic rack at 4 °C and save the supernatant (Flow-through fraction, FT).
- Wash the beads twice at 4 °C with buffer 1 supplemented with 4 mM DTT, each time rotating for 10 min in the cyclomixer and collecting and decanting the beads on the magnetic rack. Save the washes (W1 and W2 fractions).
- For an analytical application such as western blotting, elute the bound material under denaturing and reducing conditions by adding LDS (lithium dodecyl sulfate) polyacrylamide gel electrophoresis (PAGE) sample buffer with pH 8.5 to 1x and DTT to 2 mM.
- Heat the mixture at 95 °C for 5 min in a thermal block to finalize the elution.
- Collect the beads using the magnetic rack and recover the denatured eluate (E fraction) in a fresh low protein binding 1.5 mL microcentrifuge tube.
- Use the E fraction from the previous step to run a denaturing sodium dodecyl sulfate (SDS) PAGE immediately, or store the E fraction at -20 °C.
NOTE: For a preparative collection of the TAP-tag-enriched translational complexes for any subsequent application, use an alternative elution approach employing Tobacco Etch Virus (TEV) protease. Refer to the Supplementary Table 1 for further details. - To concentrate the dilute FT, W1 and W2 fractions, precipitate their material by adding 3x volumes of ice-cold acetone. Incubate the sample-acetone mix at -20 °C for 3 h.
- Pellet the precipitate by centrifuging the tubes at 13,000 x g for 10 min at 4 °C.
- Discard the supernatant and air dry the pellet in the open tubes at room temperature for 30 min.
- Dissolve the pellet in 7 µL of 1x LDS loading buffer supplemented with 2 mM DTT. Heat the samples in a thermal block set to 95 °C for 5 min.
- Load all I, FT, W1, W2, and E samples onto a 4%-12% w/v of acrylamide gradient, Bis-Tris polyacrylamide denaturing gel. Run the gel using 1x MES SDS (2- [N-mopholino]ethanesulfonic acid, sodium dodecyl sulfate) running buffer at 80 V, until the protein marker (10-250 kDa) resolves well and the lead dye reaches the bottom of the gel.
NOTE: It is recommended to load serial dilutions of the WCL (whole cell lysate) (2-10 µg) on the gel as a control. It may take several attempts to achieve comparable loading of the gel across the fraction material. - Transfer the protein content of the gel onto a polyvinylidene difluoride (PVDF) membrane by wet transfer method at 100 V for 1 h in a cold room as recommended by the western-blotting equipment manufacturer.
- Block the membrane using an appropriate blocking buffer (Phosphate Buffered Saline based) at room temperature for 1 h under constant shaking.
- Following manufacturer's instructions for antibody dilution, probe the membrane with anti-TAP-antibody for detecting the tagged eIF4A protein, anti-Pab1p antibody or anti-β-actin antibody (or any other desirable target) by overnight incubation of the membrane with Blocking Buffer (PBS)-diluted antibody (1:1,000 dilution) in a cyclomixer in a cold room.
NOTE: 1:1,000 antibody dilution is a good starting point. - Wash the membrane three times with 1x Phosphate Buffered Saline, 0.2% v/v Tween 20 (PBST) for 10 min each.
- Probe the membrane with fluorescently labeled secondary antibodies following manufacturer's instructions by incubating in a cyclomixer at room temperature for 1 h.
NOTE: 1:20,000 antibody dilution is a good starting point. - Wash the membrane three times with 1x PBST for 10 min each. Briefly rinse the membrane with deionized water, and then with absolute methanol. Dry and visualize the membrane in a fluorescent imaging system according to the manufacturer's instructions.
NOTE: Staining for other proteins can be achieved by using secondary antibodies with dyes matching different fluorescent channels (such as in the eIF4A-TAP vs. β-actin pair used here), by sequential staining or stripping and staining the same membrane or cutting the membrane from a gel loaded with repeating pattern of fractions and separately probing each piece with respective antibodies (as in the Pab1p example used here).
2. Mammalian cell protocol
- Mammalian cell culture and fixation
- In 2 T-175 flasks, grow HEK293 cells to 60%-70% confluence in Dulbecco's Modified Eagle Medium and 10% v/v Fetal Bovine Serum at 37 °C and 5% v/v carbon dioxide.
NOTE: The complete media is made by adding 55 mL of commercial FBS into a 500 mL of commercially purchased DMEM with high glucose, containing L-glutamine, phenol red and sodium bicarbonate, but no HEPES or sodium pyruvate. Cell counts per T-175 flask at 70% confluence should be in the range of 1.7-2.0 x 107. - At least 3 h prior to the desired fixation time, replace the media of the T-175 flasks with precisely 30 mL of pre-warmed complete media and replace the flasks in a cell incubator.
NOTE: Ensure that the fresh media is pipetted onto the opposite side of the flask to the cell monolayer to avoid cell detachment. Attempt to conduct the media exchange as quickly as possible, introducing minimal gas and temperature balance disturbance. - Once the cell media has been replaced, prepare buffers and chemicals required for fixation. Prepare Dulbecco's Phosphate Buffered-Saline (DPBS) with 50 mM glycine by adding 10.2 mL of 2.5 M glycine stock to a 500 mL bottle of DPBS and mixing.
- Prepare a bottle of DMEM supplemented with 10% FBS as in step 2.1.1 to be used in non-sterile conditions and a 100 mL aliquot of 0.25% Trypsin-EDTA. Source an additional bottle of commercial DPBS pre-formulated with calcium chloride (CaCl2) and magnesium chloride (MgCl2).
NOTE: The solutions can be stored at 4 °C for up to 2 weeks. - Prepare an ice box to the brim with crushed water ice such that a T-175 flask can fit evenly on top and keep in the fume hood along with the prepared buffers, also on ice.
NOTE: Due to the rapid responses of translation to any environmental change, all timings between the removal of the cell flasks from the incubator and addition of the formaldehyde solution must be minimized. - To snap chill the cells, remove the T-175 flask from the incubator and firmly press it against the ice ensuring maximal surface contact. Inside the chemical fume hood, tilt the flask onto its side so that the media collects at the side opposite to the cells. Pipette 168 µL of 37% w/v formaldehyde directly into the pooled media (to a final concentration of 0.2% w/v). Immediately mix by gently rocking the flask back and forth, close and reposition the flask on ice, ensuring it is horizontal and the cells are covered evenly.
CAUTION: Formaldehyde is a harmful substance with potential long-term adverse effects and also an irritant to both the respiratory system and skin. It should only be handled in a suitable chemical fume hood. Containers of formaldehyde must always be sealed when outside of the fume hood.
NOTE: Ensure that the formaldehyde is added directly into the cell media and not to the flask wall. Step 2.1.6 should take less than 1 min. - Incubate the flasks on ice for a further 10 min. Pour off the media into an appropriate waste container through the flask side opposite to the cells.
- Using a stripette, pipette in 30 mL of Dulbecco's Phosphate Buffered Saline without calcium and magnesium ions and additionally containing 50 mM glycine, gently on the side opposite to the cells. Mix by rocking the flask; return the flask to horizontal position and incubate for 10 min more on ice.
- Pour off the solution through the flask side opposite to the cells and gently add 7 mL of the standard 0.25% w/v Trypsin-EDTA solution to detach and resuspend the cells. Incubate the flask at room temperature for 5-10 min.
NOTE: Ensure Trypsin-EDTA solution covers all the cells evenly. Use periodic gentle tilting and rocking to promote cell detachment. - Relocate the flask vertically and using a stripette collect the detached cells by gently washing any remaining cells from the flask walls. Transfer the suspension into a 50 mL tube set on ice.
NOTE: Fixed cells can become more fragile; do not pipette intensely or more than what is required to detach the cells from the flask wall. - Immediately supplement the collected cell suspension with 20 mL of complete media (the non-sterile ice-cold media with 10% FBS) and mix by gently flipping the tube.
NOTE: The complete cell culture media (including 10% FBS) is added to neutralize the trypsin, preventing further damage to the cell membranes and cell disintegration. - Pellet the cells by centrifuging the tube at 100 x g for 5 min and 4 °C. Cell pellet must be clearly visible.
- Pour off the media and gently resuspend the cell pellet in 10 mL of ice-cold DPBS with Ca2+, Mg2+, and without glycine.
- Repeat step 2.1.12.
- Pour off the wash buffer and gently resuspend the cell pellet in 800 µL of ice-cold DPBS with Ca2+, Mg2+, without glycine, on ice. Transfer the resuspended cells into a new low protein binding 1.5 mL microcentrifuge tube.
- Centrifuge the tube at 100 x g for 3 min and 4 °C. Carefully discard the supernatant using a 1 mL pipettor. At this stage, the cell pellet can be frozen at -80 °C or proceed to the cell lysis step.
NOTE: Frozen cell pellets can be stored at -80 °C up to 1 year. We found that cell pellet freezing facilitates subsequent lysis and recommend freezing even if longer term storage is not planned.
- In 2 T-175 flasks, grow HEK293 cells to 60%-70% confluence in Dulbecco's Modified Eagle Medium and 10% v/v Fetal Bovine Serum at 37 °C and 5% v/v carbon dioxide.
- Mammalian cell disruption and cytosol collection
- In a biosafety cabinet, add 300 µL of the lysis buffer based on nonionic, nondenaturing detergent and 7 µL of 40 U/µL RNase inhibitor. Mix well by pipetting using a 1 mL tip.
- Carefully attach a 25 G needle to a 1-3 mL syringe and vigorously pipette the mixture, using at least seven slow upward intake and fast downward exhaust strokes.
- Discard the syringe and needle into a sharps bin and repeat the procedure using a 0.3 mL syringe equipped with a 31 G needle.
- Discard the syringe and needle into a sharps bin. Centrifuge the tubes at 4 °C, 12,000 x g for 5 min to pellet the cell debris.
- Transfer the supernatant into a new low protein binding 1.5 mL microcentrifuge tube. Store both, the cell debris (for control purposes) and the resultant clarified cell lysate at -80 °C.
NOTE: Optical density of the lysate ranges between 25-30 AU260 when two T-175 flasks are combined and the recommended volumes followed. The lysates and cell debris can be stored at -80 °C up to 1 year.
- Separation of the fixed (poly)ribosomal complexes from the non-translated fractions of the cytosol
- Prepare linear 15%-45% w/v sucrose gradients in 13 mL thin wall polypropylene tubes, 14 x 89 mm, using freeze-thaw method generally as described in step 1.3.1 of the yeast protocol, but using buffer 2 (Figure 2a).
NOTE: Thaw the gradients overnight in a cold room at 4 °C the night prior to the fractionation. - Load 150-250 (maximally 300) µL of the cell lysate from the previous step 2.2.5 onto the balanced gradients. Store the remaining lysate at -80 °C and use for control purposes.
NOTE: Here an example of sedimentation-based segregation into polysomal, ribosomal and 'free' SSU fractions is provided. Refer to the provided Supplementary Table 1 for an alternative approach. - Ultracentrifuge the tubes in a medium volume swing-bucket rotor at 4 °C, average g-force 178,305 x g (k-factor 143.9) for 1 h 45 min.
- 30 min prior to the spin completion, set up and baseline the gradient fractionator, as described in the yeast protocol steps 1.4.7-1.4.9.
- Fractionate the gradients generally as described in the yeast protocol steps 1.4.10-1.4.11.
NOTE: This step will separate polysomal, ribosomal and 'free' SSU fractions. Polysomal fractions may be used in polysome profiling experiments. - Immediately transfer the collected fractions on ice and if not further processed, store at -80°C up to 6 months.
NOTE: If the fraction collector tube change is synchronized with the on-line fraction identification and segregation, we recommend using up to 800 µL fractions (collection time of 32 s per fraction at 1.5 mL/min). If the fractionation is performed without using the in-line absorbance readout, it is recommended using 250-500 µL fractions (10-20 s per fraction at 1.5 mL/min). Following separation, the fractions can be used for immunopurification, electron microscopy, denaturing PAGE and western blotting straight away, or subjected to crosslink reversal for subsequent RNA and/or proteomics analyses.
- Prepare linear 15%-45% w/v sucrose gradients in 13 mL thin wall polypropylene tubes, 14 x 89 mm, using freeze-thaw method generally as described in step 1.3.1 of the yeast protocol, but using buffer 2 (Figure 2a).
Results
Translational complexes are sensitive to the ionic composition of the buffers, which is particularly important during ultracentrifugation where sedimentation properties are assessed. We thus tested several sedimentation buffers using clarified lysate extracted from ground non-fixed yeast material, in order to select conditions best suited to resolve translational complexes and separate ribosomal subunits (SSU, LSU), monosomes (RS) and polysomes across the gradient. All buffers were based on the core composition containing 25 mM HEPES-KOH pH 7.6 and 2 mM DTT. The concentrations of KCl, MgCl2, CaCl2, and EDTA were further modified across the buffers (Figure 2a), and these components were added to the lysates before gradient loading and to the sucrose gradient buffers before gradient casting, accordingly.
In buffers 1 and 2 well-resolved translational complexes were obtained. Buffer 1 resulted in somewhat better separation of the small ribosomal subunits (SSUs) (Figure 2a). Omittance of MgCl2 and addition of EDTA (buffers 3,4) caused loss of the high sedimentation properties for most of the polysomes and likely their partial disassembly (Figure 2a). While addition of 2.5 mM CaCl2 resulted in somewhat more homogeneous polysomal peaks, the improvement was marginal and the overall amount of the polysomal material decreased in this case (Figure 2a) as compared to buffers 1 and 2. We thus selected buffer 1 as the working buffer of choice.
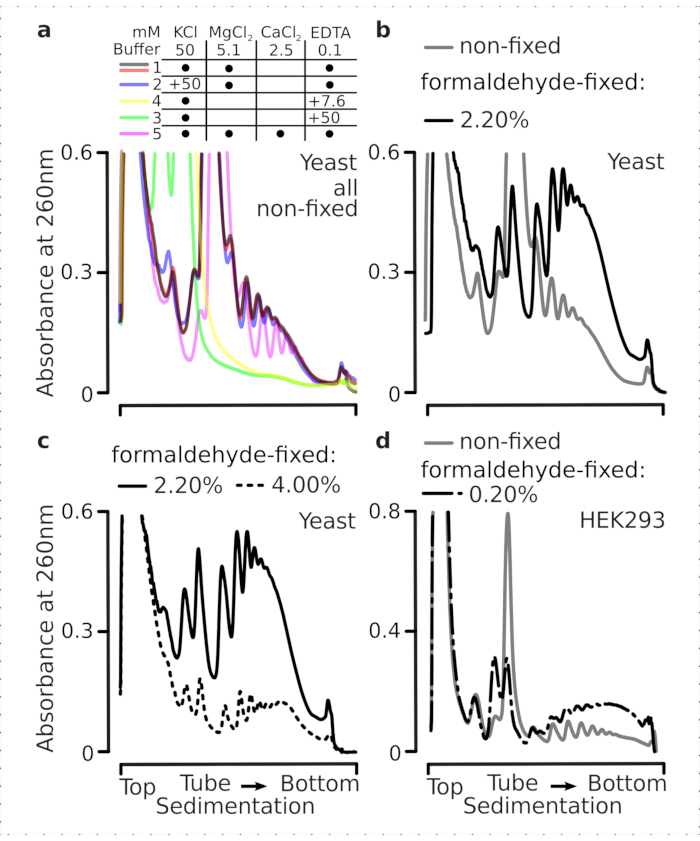
Figure 2: Buffer conditions for translational complex extraction and assessment of the stabilizing effect of the fixation. Shown are UV absorbance profiles collected at 260 nm for the total yeast cell lysate separated in 10%-40% w/v sucrose gradients. (a) Effects of mono- and divalent salts and magnesium ion sequestration on the sedimentation of material extracted from non-fixed yeast cells. Red and gray lines represent a typical replicate. (b,c) Comparison of lysates derived from non-fixed (gray line), 2.2% (black line) and 4.4% (black dotted line) w/v of formaldehyde-fixed yeast cells. (d) Stabilization of polysomes by the optimized 0.2% w/v of formaldehyde fixation (black dashed-and-dotted line) of HEK 293T cells, as compared to the material from same non-fixed cells (gray line). Please click here to view a larger version of this figure.
We next checked the effect of polysomal stabilization by fixation with different formaldehyde concentrations. Using the otherwise same cell material, buffers, cell handling and timing approaches, we compared material extracted from non-fixed cells and cells fixed with 2.2% and 4% w/v of formaldehyde (Figure 2b,c). We found that 2.2% w/v of formaldehyde was better suited for fixation as while it excellently preserved the polysomes as can be judged by the polysome-to-monosome ratio (Figure 2b), it did not reduce the overall yield of the ribosomal material compared to 4% w/v of formaldehyde, which exhibited clear signs of over-fixation (Figure 2c).
For the material derived from mammalian cells, due to the larger lysis buffer-to-cell volume ratio required by the detergent-based extraction, buffer 2 (Figure 2a) was used. This produced well-resolved translational complexes upon sedimentation in sucrose gradients (Figure 2d). Notably, a much lower concentration of formaldehyde of 0.2% w/v was used, as higher concentrations resulted in substantial polysomal and ribosomal material loss (data not shown). In similarity to the results obtained with yeast cells, crosslink-stabilized material demonstrated better preservation of the polysomes and higher polysome-to-monosome ratio (Figure 2d).
We next tested whether the selected formaldehyde fixation conditions are efficient enough to stabilize actively translated mRNA within the polysomal fractions as a result of crosslinking, and the improved polysomal yield is not just a consequence of inhibiting enzyme function and translation elongation progression. We used EDTA and high monovalent salt (KCl) to destabilize polysomes and ribosomes. These reagents were added to the clarified yeast cell lysates, and included in all subsequent buffers and sucrose gradients on top of the buffer 1 composition, respectively.
Indeed, 15 mM EDTA exhibited a lesser destabilization effect on the polysomal fractions derived from the fixed cells (Figure 3a), confirming that the crosslinked complexes are more robust. The destabilizing effects of EDTA can be somewhat overcome by increasing the concentration of formaldehyde, as material from the 4% w/v of formaldehyde-fixed cells resisted unfolding better (Figure 3a). However, increasing EDTA concentration to 50 mM resulted in destabilization of most of the translational complexes under both fixed and non-fixed conditions, as can be deduced from the slower sedimentation of the material and absence of well-shaped peaks (Figure 3b). This can be explained by the partial unfolding of structures and overall loss of compactness, rather than by the complete dissociation of polysomal components from the mRNA. Even in this case, the crosslinked material has demonstrated faster sedimentation (Figure 3b).
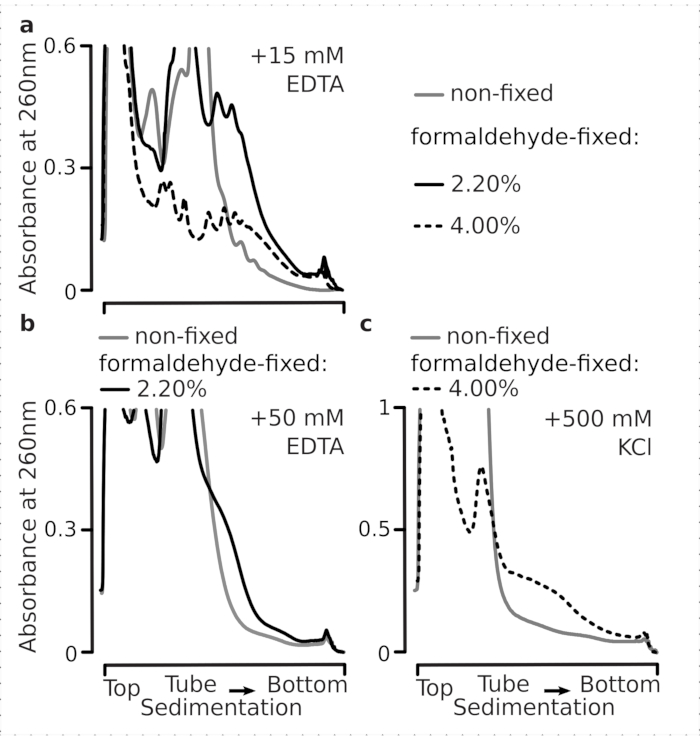
Figure 3: Effects of in vivo yeast formaldehyde fixation on the stability of polysomes. Buffer 1 (see text and Figure 2a) was used in all experiments. Data type and plotting as described in the Figure 2 legend. (a) Comparison of the addition of 15 mM EDTA to the cell lysates and subsequent buffers on the stability of the polysomes derived from non-fixed (gray line), 2.2% (black line) and 4% (black dotted line) w/v of formaldehyde-fixed cells. (b) same as (a), but for the addition of 50 mM EDTA and excluding 4% w/v of formaldehyde-fixed cells. (c) same as (a), but for the addition of 500 mM KCl and excluding 2.2% w/v of formaldehyde-fixed cells. Please click here to view a larger version of this figure.
Similar to the EDTA effects, at 500 mM KCl, we found major improvement of the stability with 4% w/v of formaldehyde fixation (Figure 3c). The apparent loss of compactness in this case can also be explained by partial detachment of the constituents of the ribosomal complexes, rather than their complete dissociation from the RNA. Overall, polysomes derived from formaldehyde-fixed cells demonstrated higher resistance to unfolding and structural destabilization, consistent with forming additional covalent bonds within these complexes.
During stimulating growth conditions, mRNAs can be rapidly initiated resulting in accumulation of multiple ribosomes on the same mRNA molecules, which form structures known as polyribosomes, or polysomes. Polysomes can be separated by ultracentrifugation in sucrose gradients, where they sediment based on their order (number of concurrently attached ribosomes on mRNA). When translation is suppressed, ribosomes fail to engage in another round of translation soon enough, resulting in (partial) 'disassembly' of polysomes, which is exhibited as a modal shift toward the polysomes of a lower order and accumulation of monosomes4,26.
A model of translational response that can be visualized on the polysome order distribution level can be provided by glucose starvation. Glucose depletion elicits one of the most dramatic and rapid translational inhibitory effects on yeast1,3,40. Previous studies evidenced that within 1 min of glucose depletion, loss of polysomes, accumulation of monosomes and inhibition of translation initiation can occur4. Within 5 min of glucose re-supplement, translation is quickly restored with evident increase in polysomes3,4. It was also observed that translation was inhibited when cells were exposed to media containing glucose of 0.5% (w/v) or lower and there was no effect seen in glucose levels of 0.6% (w/v) or higher.
We thus wished to determine whether our fixation conditions are suitable for the preservation of the translational differences within the dynamics of glucose stress response, as can be assessed by the polysome-to-monosome ratio. We compared the material from the cells grown in mid-exponential phase on high glucose (2.00% w/v added) with those transferred for 10 min into media with no or low added (0.00% or 0.25% w/v, respectively) glucose. The fixation has been performed using 2.2% w/v of formaldehyde in parallel in the control (non-starved; rapid media replacement with same standard media containing 2% w/v added glucose, followed by incubation for 10 min and fixation) and 10 min starved (rapid media replacement with same media but low 0.25 w/v or no added glucose, followed by incubation for 10 min and fixation) cells.
Consistent with the earlier findings, we observed that yeast cells heavily suppress translation upon glucose starvation stress (Figure 4a). Both, no added and low glucose conditions induced polysome disassembly, with slightly but evidently more polysomes retained in the case of low added glucose. Thus, the yeast glucose removal response may be not of an all-on or all-off type and is gradually tuned. Affirming expectations for the stabilizing action of the formaldehyde crosslinking, polysomal material from the fixed cells has demonstrated a higher distinction between the starved and non-starved cells, arguably preserving a higher dynamic range of the response (Figure 4b). Intriguingly, in the case of material from the fixed cells, low added glucose concentration resulted in the specific polysomal abundance that is much better differentiated from the no added glucose condition, compared to the non-fixed cells (Figure 4a). This is a strong indication of the suitability of formaldehyde fixation approach in preserving and capturing relatively minute and transient differences in the equilibrium of highly dynamic processes, such as during translational responses.

Figure 4: Capturing rapid changes in yeast translation upon glucose starvation. Buffer 1 (see text and Figure 2a) was used in all experiments. Data type and plotting as described in the Figure 2 legend. (a) Cell lysates obtained from non-starved (gray line), restricted glucose-starved (0.25% w/v added glucose for 10 min; brown line) and glucose-depleted (no added glucose for 10 min; red line) non-fixed yeast cells. (b) same as (a), but for 2.2% w/v formaldehyde-fixed cells. Please click here to view a larger version of this figure.
Monitoring translational status by the ribosomes associated with actively translating mRNA using sucrose gradient sedimentation ('polysome profiling') is a widely applied technique26,27,28. In combination with quantitative microarray analysis and more recently with high throughput sequencing28,44, polysome profiling provides information about ribosome-associated mRNAs transcriptome-wide. With several assumptions, it has been traditionally argued in the field of protein biosynthesis research that the polysomal presence is an indication of active involvement in translation of the respective mRNAs. A further conclusion is often (but not always) justified, that the more ribosomes are present on an mRNA of a given length (the higher the order of the polysomes), the more actively that mRNA is involved in translation. Thus, separating the polysomal fraction from the rest of material can be useful from the standpoint of isolating the actively translated RNA. Within the footprint profiling approaches, and particularly TCP-seq10,38,39 that generates a separate population of the liberated SSUs derived from the scanning, start and stop codon complexes, it may be additionally insightful to remove ribosomal subunits that do not co-sediment with the complete monosomes or polysomes.
We thus have employed separation of the 'non-translated' mRNPs such as free SSUs (mRNA bound to single SSU or SSUs without attached mRNA) away from the 'actively translating' pool of mRNAs. To achieve this, we assumed that mRNAs involved in interactions with either one (mono-) or several ribosomes (polysomes) can be actively translated. Such complexes can be separated from the others by their higher sedimentation coefficient. We also suggested to separate the 'actively translated' pool of mRNAs into a sucrose cushion (50% w/v of sucrose) instead of direct pelleting the material on the tube wall. Centrifugation of the fast-sedimenting complexes into the cushion allowed us to monitor the separation using absorbance profile readout and to achieve a higher output of the solubilized, non-aggregated and non-denatured material, compared to pelleting and re-solubilization10,38.
Overall, to purify the individual SSUs, ribosomes, disomes, and potentially compactly packed polysomes of a higher order, fixed clarified lysates were subjected to a two-stage ultracentrifugation process (Figure 5). In the first sucrose gradient, the ultracentrifugation resulted in separated free SSUs and LSUs in the top (10%-20% w/v of sucrose) portion of the gradient, whereas the crosslinked translated pool including polysomes and mRNAs associated with one complete ribosome were concentrated at the bottom (50% w/v of sucrose) of the gradient (Figure 5a). The bottom 50% w/v of sucrose layer containing the translated mRNA pool was then concentrated and its RNA digested with RNase I, followed by a second sucrose gradient ultracentrifugation to obtain separate SSU, LSU, RS, RNase resistant disomes (DS) and minor fraction of higher-order nuclease-resistant polysomes (Figure 5b). Negative staining with uranyl acetate and imaging with a transmission electron microscope confirmed the identity of the complexes isolated in each sedimentation stage (Figure 5).

Figure 5: Isolation of the total translated RNA fractions away from the untranslated RNA. (a,c) Schematic (left) and the respective representative results (right; data type and plotting as described in the Figure 2 legend) of (a) first discontinuous sucrose gradient separation of the non-translated cytosol fractions including free SSUs and the translated mRNA pool identified by co-sedimentation with ribosomes and polysomes, and (c) separation of the individual ribosomal complexes liberated from the translated mRNA pool by controlled RNase I digestion and ultracentrifugation through a second linear sucrose gradient into SSU, LSU, ribosomal (RS), and nuclease-resistant disomal (DS) fractions. High (15 AU260) and low (8 AU260) amounts of the non-starved digested material were included to demonstrate a possibility of increasing the ultracentrifugation loads when minor fractions are of an interest. Higher-order nuclease-resistant polysomes can also be identified (e.g., trisomes in the provided examples). (b,d) Representative TEM images of uranyl acetate-contrasted fractions from (a,c), respectively as labeled. Please click here to view a larger version of this figure.
In order to check the suitability of the fixation regimen for the retention of transient ribosome-associated proteins (particularly, eIFs), we tested for the co-sedimentation of eIF4A, a labile eIF dynamically bound to the ribosome, across the ribosomal fractions. We took advantage of the eIF4A Tandem Affinity Purification (TAP) tagged yeast strain (TIF1-TAP) and investigated eIF4A presence in material derived from the fixed vs. non-fixed cells by using anti-TAP antibody, compared to the abundance of Pab1p as an additional RNA-binding control, using SDS-PAGE followed by western blotting (Figure 6).
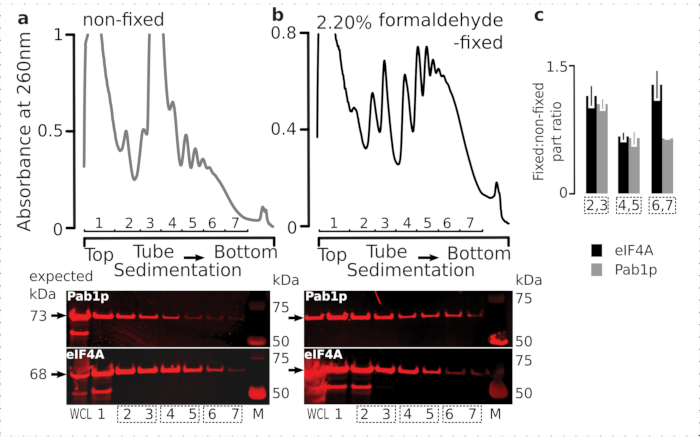
Figure 6: Stabilization of transient proteins in the translational complexes upon in vivo formaldehyde fixation. (a,b) (top plots) Whole cell lysate (WCL) of (a) non-fixed and (b) 2.2% formaldehyde-fixed eIF4A-TAP yeast cells separated by ultracentrifugation and visualized as described in the Figure 2 legend. (bottom plots) Western-blot imaging of the respective sucrose gradient fractions upon separation of the material analyzed in the corresponding gradients (top plots), and WCL as a control. (c) Average ratio between the eIF4A or Pab1p abundance in the fractions of fixed and non-fixed material. Relative proportions (normalized to the signal of all 2-7 fractions) of eIF4A (black bars) and Pab1p (gray bars) were calculated across 2,3 (SSU, LSU), 4,5 (RS, light polysomes), and 6,7 (heavy polysomes) from the data of (a,b) (bottom plots), and their fixed to non-fixed ratio taken. Error bars indicate standard deviation of the ratio from the mean with the pooled fractions (dotted boxes) treated as replicates. Please click here to view a larger version of this figure.
Consistent with their high abundance in the cells, we observed a high intensity of the signal from both of the proteins in the whole cell lysate (WCL) and slower-sedimenting fractions derived from non-fixed cells (Figure 6a, bottom panel). We have also detected substantial amounts of these proteins in the WCL derived from the fixed cells and reassuring the efficiency of the crosslinked material extraction and absence of unexpected losses (Figure 6b, bottom panel). However, in contrast to the non-fixed cells, material from the fixed cells demonstrated elevated relative presence of eIF4A in the faster-sedimenting ribosomal fractions, in comparison to Pab1p (Figure 6c). This result suggests that eIF4A remains more firmly associated with the polysomes in formaldehyde-crosslinked material.
Having confirmed the positive and specific stabilization effect of crosslinking on eIF4A presence in the ribosomal fractions, we used the fixed material from eIF4A-tagged (TIF1-TAP) yeast strain to capture and enrich eIF4A-containing complexes by affinity purification with magnetic IgG beads. We have affinity-enriched WCL, free SSU and polysomal (translated mRNA pool) fractions after the first sedimentation through sucrose gradient (e.g., section 1.3 of the yeast protocol), as well as SSU, LSU and RS fractions from the second sedimentation upon the disassembly of the translated pool into individual complexes with RNase I (e.g., section 1.4 of the yeast protocol) (Figure 7). In all cases, except for the LSU fraction, we were able to observe selective enrichment of the eIF4A in the purified fractions (eluate, E), in comparison to the presence of β-actin in the source material (input, I) (Figure 7).
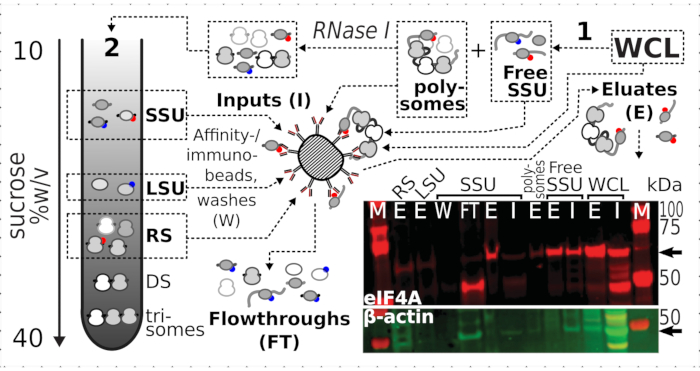
Figure 7: Selective immunopurification of in vivo formaldehyde-stabilized translational complexes by transiently associated eIF4A. The schematic illustrates the source of different translational complexes and eIF4A epitope, including the non-fractionated clarified WCL of the eIF4A-TAP yeast cells; free SSUs and translated RNA pool (polysomes) segregated in the first ultracentrifugation; SSU, LSU and RS fractions liberated from the translated RNA by RNase I digestion and segregated using second ultracentrifugation (see text). Western blot image provides a visualization of the eIF4A abundance in the fractions compared to the abundance of concurrently stained β-actin control. Please click here to view a larger version of this figure.
Supplementary Table 1. Please click here to download this Table.
Discussion
Formaldehyde fixation is a convenient and popular method of achieving rapid in vivo crosslinking of biomolecules10,36,45,46,47,48. Compared to the other potential biomolecule targets, successful capture of translational complexes necessitates an immediate fixation during the snap chilling of the cells or other material. Without the undelayed stabilization, there is a potential for different translation-related processes to continue, shifting the complex distribution away from the unperturbed in vivo state49. Compared to the other methods of translational arrest and ribosomal complex stabilization, the swiftness of formaldehyde action across cell membranes and the indiscriminate nature of the crosslinks promise preservation of the maximal diversity of the translation complex intermediates closer to their natively distributed states50.
The approach presented here has been established and optimized in both yeast and mammalian cells, and methods have now been derived by other groups for use across more diverse biological material, such as in whole vertebrates (e.g., zebrafish embryos)10,38,39,49,51,52. Although these works collectively reassure the versatility and broad applicability of the approach, rapid formaldehyde crosslinking of translational complexes can be considered somewhat difficult to transpose to new types of biological material due to the need of optimizations and adjustments.
A foremost requirement to the success of the method is the re-optimization of the concentration of the formaldehyde and the cell collection and disruption technique. Less permeable, small and round yeast cells require much higher (at least, 10-fold) formaldehyde concentration and physical disruption of the fixed cells. In contrast, large and flattened adherent mammalian cells in culture can be easily over-fixed and require gentle handling upon fixation, while the extraction of the fixed complexes can be performed chemically with membrane disruption using detergents. Under-crosslinking may allow less stable or more short-lived intermediates to dissociate or leak into a later state. Over-crosslinking may negatively affect the ability to isolate and study ribosomal fractions and can create selective biases such as deeper depletion of heavy complexes. In our observation, even minor alterations, such as the type of adherent human cells used, can affect the yield of the recovered crosslinked complexes and may require re-optimization of the crosslinking regimen. We can also anticipate that cells with substantially different permeability properties, such as plant cells, will require additional extensive optimization of the fixation conditions52. Yet, it is difficult to imagine a type of biological material that would be entirely incompatible with the approach.
One consideration pertinent to the mammalian fixation protocol is the density and amount of cell material used as input. It is recommended to have the cells continuously growing without re-seeding or other perturbations for at least 2 days to avoid external influences on cellular translation dynamics. Applicable for most cell types, but for the majority of adherent cells consistently achieved confluence levels of no more than 70% will ensure absence of major contact inhibition effects that can negatively and unpredictably affect translation rates.
Another interesting, and potentially uniquely convenient, feature of formaldehyde fixation stemming from its indiscriminate reactivity is the stabilization effect on translational complexes in systems of mixed taxonomy. Bacterial, and even more so translational complexes of mitochondria, chloroplasts and different intracellular parasites, have been notoriously difficult to target with specific translation inhibitors. In contrast, in the TCP-seq data, footprints mapping to the mitotranscriptome are readily observable in the data38,39,50. An interesting subsequent development could be the use of the approach to investigate translation in entire microcommunities, such as in soil, water or gut samples, where reliable rapid translational arrest and complex stabilization with any other means would be problematic.
It should also be mentioned that for the most complicated material (such as hard and/or bulky tissues), nothing prevents the use of formaldehyde stabilization immediately upon cell disruption and material homogenization. This approach is already frequently employed to remove the cell entry delay when stabilizing translational complexes with specific small molecule inhibitors33,53,54,55. Given that formaldehyde fixation has been traditionally used with excellent results for ex vivo/in vitro sample stabilization in applications such as electron microscopy45,56,57,58, we can expect even less negative effects in this case, particularly those associated with the poor extraction of the translational complexes from the thoroughly fixed cells.
Our findings confirm the usability of rapid formaldehyde fixation to stabilize highly transient complexes, such as those that include eIF4A. It is noteworthy that in contrast to mammals, yeast eIF4A is much more weakly associated with the cap binding complex eIF4F and, as a result, translational complexes in general. eIF4A is usually lost during any extensive purification of the ribosomal material in yeast29,59,60,61,62,63. Yet, in the in vivo-fixed yeast material, it is possible to achieve reliable enrichment of eIF4A in all fractions of translational complexes where its presence would be anticipated. The previously published Sel-TCP-seq data have demonstrated the enrichment of eIF2 and eIF3 that more strongly associate with the ribosomes (but also revealed transiently occurring co-translational protein complex assembly)39. Thus, the method is suitable for the detection of both, stronger and weaker attached constituents of the translational complexes.
To summarize, we have presented an approach useful to gain insights primarily into the changes occurring at the initiation phase of translation and when minimally perturbed ribosomal distribution over the mRNA is required. Importantly, the approach is suitable for the stabilization of relatively labile and dynamic components of translational complexes, such as eIF4A, and can be used broadly subjected to necessary optimizations. We have also provided evidence of the usefulness of formaldehyde fixation in the scenarios of rapid dynamic change of translation, opening up areas of investigation such as fast-paced cellular responses to environmental changes or stress conditions.
Disclosures
The authors declare no conflicts of interest.
Acknowledgements
This work was supported by Australian Research Council Discovery Project grant (DP180100111 to T.P. and N.E.S), National Health and Medical Research Council Investigator Grant (GNT1175388 to N.E.S.) and Research Fellowship (APP1135928 to T.P.). The authors acknowledge the facilities of Microscopy Australia at the Centre for Advanced Microscopy, Australian National University, a facility that is funded by the University and the Federal Government.
Materials
| Name | Company | Catalog Number | Comments |
| Yeast extract | Merck, Sigma-Aldrich | 70161 | |
| Peptone | Merck, Sigma-Aldrich | 70178 | |
| D-Glucose (Dextrose) | Merck, Sigma-Aldrich | 49139 | |
| Adenine sulphate | Amresco | 0607-50G | |
| Formaldehyde solution | Merck Sigma-Aldrich | F11635-500ML | ACS reagent, 37 wt. % in H2O, contains 10-15% Methanol as stabiliser (to prevent polymerisation) |
| RNaseOUT™ Recombinant Ribonuclease Inhibitor | Invitrogen™ byThermo Fischer Scientific | 10777019 | |
| cOmplete™, EDTA-free Protease Inhibitor Cocktail | COEDTAF-RO Roche by Merck | 11873580001 | |
| Magnesium chloride solution | (Merck/Sigma-Aldrich) | M1028 | |
| Ethylenediaminetetraacetic acid solution | (Merck/Sigma-Aldrich) | E7889 | |
| Ambion™ RNase I, cloned, 100 U/µL | Ambion | AM2294 | |
| SUPERase•In™ RNase Inhibitor (20 U/μL) | Invitrogen™ by Thermo Fisher Scientific | AM2694 | |
| Acidic phenol:chlorophorm:isoamyl alcohol 125:24:1 (pH 4.0-5.0) | (Merck/Sigma-Aldrich) | P1944-100ML | |
| Dynabeads™ Goat Anti-Mouse IgG | Invitrogen™ by Thermo Fisher Scientific) | 11033 | |
| Sodium Acetate (3 M), pH 5.5 | Invitrogen™ by Thermo Fisher Scientific) | AM9740 | |
| Glycogen (5 mg/ml) | Invitrogen™ by Thermo Fisher Scientific) | AM9510 | |
| Ethyl alcohol, Pure | Merck; Sigma Aldrich | E7023 | |
| Amersham™ Hybond® P Western blotting membranes, PVDF | Merck | GE10600023 | PVDF membrane for western blotting |
| Bolt™ 4 to 12%, Bis-Tris, 1.0 mm, Mini Protein Gel | Invitrogen™ by ThermoFischer Sientific | NW04120BOX | Protein gel |
| 4X Bolt™ LDS Sample Buffer | Invitrogen™ by ThermoFischer Sientific | B0007 | LDS sample loading buffer |
| Precision Plus Protein™ Kaleidoscope™ Prestained Protein Standards | BioRad | 1610375 | Protein ladder |
| 20X Bolt™ MES SDS Running Buffer | ThermoFischer Scientific | B0002 | PAGE runninjg buffer |
| Intercept® (PBS) Blocking Buffer | LI-COR | 927-70001 | Odyssey Blcoking buffer (PBS) |
| IRDye® 800CW Goat anti-Mouse IgG Secondary Antibody | LI-COR | 92632210 | |
| IRDye® 800CW Goat anti-Rabbit IgG Secondary Antibody | LI-COR | 92632211 | |
| TAP Tag Polyclonal Antibody | Invitrogen™ by ThermoFischer Sientific | CAB1001 | |
| Anti-beta Actin antibody | Abcam | ab8227 | |
| Sucrose | (Merck/Sigma-Aldrich) | 84097 | BioUltra, for molecular biology, ≥99.5% (HPLC) |
| DL-Dithiothreitol solution | (Merck/Sigma-Aldrich) | 43816 | BioUltra, for molecular biology, ~1 M in H2O |
| Terumo Syringe 1CC/mL | Terumo Syringe | 878499 | |
| Potassium chloride | (Merck/Sigma-Aldrich) | 60128 | |
| HEPES | (Merck/Sigma-Aldrich) | H3375 | |
| Dulbecco's Modified Eagle's Medium - high glucose | Sigma Aldrich | D5796 | |
| Fetal Bovine Serum | Sigma Aldrich | 12003C | |
| Trypsin-EDTA (0.05%), phenol red | Gibco | 25300062 | |
| Dulbecco's Phosphate Buffered Saline with Calcium and magnesium | Sigma-Aldrich | D8662 | |
| Glycine | Sigma-Aldrich | G7126 | |
| Tris hydrochloride | Merck/Sigma-Aldrich | 10812846001 | |
| Sodium dodecyl sulfate | Merck/Sigma-Aldrich | 436143 | |
| IGEPAL CA-630 | Merck/Sigma-Aldrich | I3021 | |
| Rnasin Ribonuclease Inhibitor | Promega | N2111 | |
| Stainless steel grinding jar | Retsch | 02.462.0059 | |
| MM400 mixer mill | Retsch | 20.745.0001 | |
| Gradient Fractionator | Brandel | BRN-BR-188 | |
| Thermomixer R | Eppendorf | Z605271 | |
| Nanodrop spectrophotometer | Thermo Fisher Scientific | ND-2000 | |
| 0.5-ml microcentrifuge tubes with locking devices | Eppendorf Safe-Lock | 30121023 | |
| Mini Gel Tank | (Thermo Fisher Scientific) | A25977 | PAGE running tank |
| 5 mL, Open-Top Thinwall Ultra-Clear Tube, 13 x 51mm | Beckman-Coulter | 344057 | |
| 13.2 mL, Certified Free Open-Top Thinwall Polypropylene, 14 x 89mm - 50Pk | Beckman-Coulter | 331372 | |
| Amicon Ultra-0.5 ultrafiltration devices | Merck | UFC5030 | Ultracel-30 regenerated cellulose membrane, 0.5 mL sample volume |
| Thermo Sorvall Evolution RC Floor Super Speed Centrifuge | Cambridge Scientific | 15566 | |
| Beckman Coulter Optima L-90K | GMI | 8043-30-1191 | |
| Nunc EasYFlask 175cm2 | Thermofisher Scientific | 159910 | |
| Falcon 50 mL Conical Centrifuge Tubes | Thermofisher Scientific | 14-432-22 | |
| 25 mL Serological Pipette | Sigma-Aldrich | SIAL1250 | |
| 10 mL Serological Pipette | Sigma-Aldrich | SIAL1100 | |
| DNA lobind tubes | Eppendorf | 30108051 | |
| Cold Centrifuge 5810 R | Eppendorf | EP022628188 | for 50 mL tubes |
| Orbital Shaking Incubator | Ratek | OM11 | |
| Frezco 17 Microcentrifuge | Thermofisher Scientific | 75002402 | |
| Eppendorf DNA lo-bind tubes | Merck/Sigma-Aldrich | EP0030108051 | |
| Eppendorf® Protein LoBind tubes | Merck/Sigma-Aldrich | EP0030108116 | |
| SW 41 Ti Swinging bucket rotor | Beckman-Coulter | 331362 | |
| Heracell™ 150i CO2 Incubator, 150 L | Thermofisher Scientific | 51026282 | |
| 0,3 mL ultra-fine II short insulin syringe | BD Medical | 328822 | |
| 3 mL syringe with Luer Lok tip | BD Medical | 302113 | |
| 25 G x 16 mm Hypodermic Needle | Terumo | TUAN2516R1 |
References
- Janapala, Y., Preiss, T., Shirokikh, N. E. Control of translation at the initiation phase during glucose starvation in yeast. International Journal of Molecular Sciences. 20 (16), 4043 (2019).
- Masvidal, L., Hulea, L., Furic, L., Topisirovic, I., Larsson, O. mTOR-sensitive translation: Cleared fog reveals more trees. RNA Biology. 14 (10), 1299-1305 (2017).
- Ashe, M. P., De Long, S. K., Sachs, A. B. Glucose depletion rapidly inhibits translation initiation in yeast. Molecular Biology of the Cell. 11 (3), 833-848 (2000).
- Crawford, R. A., Pavitt, G. D. Translational regulation in response to stress in Saccharomyces cerevisiae. Yeast. 36 (1), 5-21 (2019).
- Melamed, D., Pnueli, L., Arava, Y. Yeast translational response to high salinity: global analysis reveals regulation at multiple levels. RNA. 14 (7), 1337-1351 (2008).
- Hershey, J. W., Sonenberg, N., Mathews, M. B. Principles of translational control: An overview. Cold Spring Harbor Perspectives in Biology. 4 (12), 011528 (2012).
- Mata, J., Marguerat, S., Bähler, J. Post-transcriptional control of gene expression: a genome-wide perspective. Trends in Biochemical Sciences. 30 (9), 506-514 (2005).
- Spriggs, K. A., Bushell, M., Willis, A. E. Translational regulation of gene expression during conditions of cell stress. Molecular Cell. 40 (2), 228-237 (2010).
- Liu, B., Qian, S. B. Translational reprogramming in cellular stress response. Wiley Interdisciplinary Reviews RNA. 5 (3), 301-315 (2014).
- Archer, S. K., Shirokikh, N. E., Beilharz, T. H., Preiss, T. Dynamics of ribosome scanning and recycling revealed by translation complex profiling. Nature. 535 (7613), 570-574 (2016).
- Hinnebusch, A. G., Ivanov, I. P., Sonenberg, N. Translational control by 5'-untranslated regions of eukaryotic mRNAs. Science. 352 (6292), 1413-1416 (2016).
- Dever, T. E., Green, R. The elongation, termination, and recycling phases of translation in eukaryotes. Cold Spring Harbor Perspectives in Biology. 4 (7), 013706 (2012).
- Shirokikh, N. E., Preiss, T. Translation initiation by cap-dependent ribosome recruitment: Recent insights and open questions. Wiley Interdisciplinary Reviews RNA. 9 (4), 1473 (2018).
- Jiménez-Díaz, A., Remacha, M., Ballesta, J. P., Berlanga, J. J. Phosphorylation of initiation factor eIF2 in response to stress conditions is mediated by acidic ribosomal P1/P2 proteins in Saccharomyces cerevisiae. PLoS One. 8 (12), 84219 (2013).
- Sonenberg, N., Hinnebusch, A. G. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell. 136 (4), 731-745 (2009).
- Majmundar, A. J., Wong, W. J., Simon, M. C. Hypoxia-inducible factors and the response to hypoxic stress. Molecular Cell. 40 (2), 294-309 (2010).
- Barraza, C. E., et al. The role of PKA in the translational response to heat stress in Saccharomyces cerevisiae. PLoS One. 12 (10), 0185416 (2017).
- Richter, K., Haslbeck, M., Buchner, J. The heat shock response: Life on the verge of death. Molecular Cell. 40 (2), 253-266 (2010).
- Jamar, N. H., Kritsiligkou, P., Grant, C. M. The non-stop decay mRNA surveillance pathway is required for oxidative stress tolerance. Nucleic Acids Research. 45 (11), 6881-6893 (2017).
- Chen, Z., et al. The complete pathway for thiosulfate utilization in Saccharomyces cerevisiae. Applied and Environmental Microbiology. 84 (22), (2018).
- Marzluf, G. A. Molecular genetics of sulfur assimilation in filamentous fungi and yeast. Annual Review of Microbiology. 51, 73-96 (1997).
- Miller, D., Brandt, N., Gresham, D. Systematic identification of factors mediating accelerated mRNA degradation in response to changes in environmental nitrogen. PLoS Genetics. 14 (5), 1007406 (2018).
- Zhang, W., Du, G., Zhou, J., Chen, J. Regulation of sensing, transportation, and catabolism of nitrogen sources in Saccharomyces cerevisiae. Microbiology and Molecular Biology Reviews. 82 (1), (2018).
- Tokpohozin, S. E., Fischer, S., Becker, T. Selection of a new Saccharomyces yeast to enhance relevant sorghum beer aroma components, higher alcohols, and esters. Food Microbiology. 83, 181-186 (2019).
- Walker, G. M., Stewart, G. G. Saccharomyces cerevisiae in the production of fermented beverages. Beverages. 2 (4), 30 (2016).
- Chassé, H., Boulben, S., Costache, V., Cormier, P., Morales, J. Analysis of translation using polysome profiling. Nucleic Acids Research. 45 (3), 15 (2017).
- Jin, H. Y., Xiao, C. An integrated polysome profiling and ribosome profiling method to investigate in vivo translatome. Methods in Molecular Biology. 1712, 1-18 (2018).
- Arava, Y., et al. Genome-wide analysis of mRNA translation profiles in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences of the United States of America. 100 (7), 3889-3894 (2003).
- Lackner, D. H., et al. A network of multiple regulatory layers shapes gene expression in fission yeast. Molecular Cell. 26 (1), 145-155 (2007).
- Ingolia, N. T., Ghaemmaghami, S., Newman, J. R. S., Weissman, J. S. Genome-Wide Analysis in Vivo of Translation with Nucleotide Resolution Using Ribosome Profiling. Science. 324 (5924), 218-223 (2009).
- Ingolia, N. T., Hussmann, J. A., Weissman, J. S. Ribosome Profiling: Global Views of Translation. Cold Spring Harbor Perspectives in Biology. 11 (5), (2019).
- Gerashchenko, M. V., Lobanov, A. V., Gladyshev, V. N. Genome-wide ribosome profiling reveals complex translational regulation in response to oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 109 (43), 17394-17399 (2012).
- Hussmann, J. A., Patchett, S., Johnson, A., Sawyer, S., Press, W. H. Understanding biases in ribosome profiling experiments reveals signatures of translation dynamics in yeast. Proceedings of the National Academy of Sciences Genetics. 11 (12), 1005732 (2015).
- Santos, D. A., Shi, L., Tu, B. P., Weissman, J. S. Cycloheximide can distort measurements of mRNA levels and translation efficiency. Nucleic Acids Research. 47 (10), 4974-4985 (2019).
- Schneider-Poetsch, T., et al. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nature Chemical Biology. 6 (3), 209-217 (2010).
- Hoffman, E. A., Frey, B. L., Smith, L. M., Auble, D. T. Formaldehyde crosslinking: A tool for the study of chromatin complexes. Journal of Biological Chemistry. 290 (44), 26404-26411 (2015).
- Kage, U., Powell, J. J., Gardiner, D. M., Kazan, K. Ribosome profiling in plants: What is not lost in translation. Journal of Experimental Botany. 71 (18), 5323-5332 (2020).
- Shirokikh, N. E., Archer, S. K., Beilharz, T. H., Powell, D., Preiss, T. Translation complex profile sequencing to study the in vivo dynamics of mRNA-ribosome interactions during translation initiation, elongation and termination. Nature Protocols. 12 (4), 697-731 (2017).
- Wagner, S., et al. Selective translation complex profiling reveals staged initiation and co-translational assembly of initiation factor complexes. Molecular Cell. 79 (4), 546-560 (2020).
- Zlotorynski, E. Profiling ribosome dynamics. Nature Reviews Molecular Cell Biology. 17 (9), 535-535 (2016).
- Sen, N. D., Gupta, N., S, K. A., Preiss, T., Lorsch, J. R., Hinnebusch, A. G. Functional interplay between DEAD-box RNA helicases Ded1 and Dbp1 in preinitiation complex attachment and scanning on structured mRNAs in vivo. Nucleic Acids Research. 47 (16), 8785-8806 (2019).
- Zhao, J., Qin, B., Nikolay, R., Spahn, C. M. T., Zhang, G. Translatomics: The global view of translation. International Journal of Molecular Sciences. 20 (1), 20010212 (2019).
- Luthe, D. S. A simple technique for the preparation and storage of sucrose gradients. Analytical Biochemistry. 135 (1), 230-232 (1983).
- Wang, Z., Gerstein, M., Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nature Review Genetics. 10 (1), 57-63 (2009).
- Orlando, V. Mapping chromosomal proteins in vivo by formaldehyde-crosslinked-chromatin immunoprecipitation. Trends in Biochemical Sciences. 25 (3), 99-104 (2000).
- Schmiedeberg, L., Skene, P., Deaton, A., Bird, A. A Temporal Threshold for Formaldehyde Crosslinking and Fixation. PLoS One. 4 (2), 4636 (2009).
- Solomon, M. J., Varshavsky, A. Formaldehyde-mediated DNA-protein crosslinking: A probe for in vivo chromatin structures. Proceedings of the National Academy of Sciences. 82 (19), 6470-6474 (1985).
- Solomon, M. J., Larsen, P. L., Varshavsky, A. Mapping proteinDNA interactions in vivo with formaldehyde: Evidence that histone H4 is retained on a highly transcribed gene. Cell. 53 (6), 937-947 (1988).
- Bohlen, J., Fenzl, K., Kramer, G., Bukau, B., Teleman, A. A. Selective 40S footprinting reveals cap-tethered ribosome scanning in human cells. Molecular Cell. 79 (4), 561-574 (2020).
- Shirokikh, N. E. Translation complex stabilization on messenger RNA and footprint profiling to study the RNA responses and dynamics of protein biosynthesis in the cells. Critical Reviews in Biochemistry and Molecular Biology. , (2021).
- Giess, A., et al. Profiling of small ribosomal subunits reveals modes and regulation of translation initiation. Cell Reports. 31 (3), 107534 (2020).
- Firmino, A. A. P., et al. Separation and paired proteome profiling of plant chloroplast and cytoplasmic ribosomes. Plants (Basel). 9 (7), (2020).
- Gerashchenko, M. V., Gladyshev, V. N. Translation inhibitors cause abnormalities in ribosome profiling experiments. Nucleic Acids Research. 42 (17), 134 (2014).
- Santos, D. A., Shi, L., Tu, B. P., Weissman, J. S. Cycloheximide can distort measurements of mRNA levels and translation efficiency. Nucleic Acids Research. 47 (10), 4974-4985 (2019).
- Schneider-Poetsch, T., et al. Inhibition of eukaryotic translation elongation by cycloheximide and lactimidomycin. Nature Chemical Biology. 6 (3), 209-217 (2010).
- Plénat, F., et al. Formaldehyde fixation in the third millennium. Annales De Pathologie. 21 (1), 29-47 (2001).
- Salic, A., Mitchison, T. J. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proceedings of the National Academy of Sciences. 105 (7), 2415-2420 (2008).
- Wang, N. S., Minassian, H. The formaldehyde-fixed and paraffin-embedded tissues for diagnostic transmission electron microscopy: A retrospective and prospective study. Human Pathology. 18 (7), 715-727 (1987).
- Grifo, J. A., et al. Characterization of eukaryotic initiation factor 4A, a protein involved in ATP-dependent binding of globin mRNA. Journal of Biological Chemistry. 257 (9), 5246-5252 (1982).
- Li, Y. Commonly used tag combinations for tandem affinity purification. Biotechnology and Applied Biochemistry. 55 (2), 73-83 (2010).
- Blum, S., et al. ATP hydrolysis by initiation factor 4A is required for translation initiation in Saccharomyces cerevisiae. Proceedings of the National Academy of Sciences. 89 (16), 7664-7668 (1992).
- Merrick, W. C. eIF4F: A Retrospective. Journal of Biological Chemistry. 290 (40), 24091-24099 (2015).
- Rogers, G. W., Komar, A. A., Merrick, W. C. eIF4A: The godfather of the DEAD box helicases. Progress in Nucleic Acid Research and Molecular Biology. 72, 307-331 (2002).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved