A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
In Vitro Drug Screening Against All Life Cycle Stages of Trypanosoma cruzi Using Parasites Expressing β-galactosidase
In This Article
Summary
We describe a high-throughput colorimetric assay measuring β-galactosidase activity in three life cycle stages of Trypanosoma cruzi, the causative agent of Chagas disease. This assay can be used to identify trypanocidal compounds in an easy, fast, and reproducible manner.
Abstract
Trypanosoma cruzi is the causative agent of Chagas disease (ChD), an endemic disease of public health importance in Latin America that also affects many non-endemic countries due to the increase in migration. This disease affects nearly 8 million people, with new cases estimated at 50,000 per year. In the 1960s and 70s, two drugs for ChD treatment were introduced: nifurtimox and benznidazole (BZN). Both are effective in newborns and during the acute phase of the disease but not in the chronic phase, and their use is associated with important side effects. These facts underscore the urgent need to intensify the search for new drugs against T. cruzi.
T. cruzi is transmitted through hematophagous insect vectors of the Reduviidae and Hemiptera families. Once in the mammalian host, it multiplies intracellularly as the non-flagellated amastigote form and differentiates into the trypomastigote, the bloodstream non-replicative infective form. Inside the insect vector, trypomastigotes transform into the epimastigote stage and multiply through binary fission.
This paper describes an assay based on measuring the activity of the cytoplasmic β-galactosidase released into the culture due to parasites lysis by using the substrate, chlorophenol red β-D-galactopyranoside (CPRG). For this, the T. cruzi Dm28c strain was transfected with a β-galactosidase-overexpressing plasmid and used for in vitro pharmacological screening in epimastigote, trypomastigote, and amastigote stages. This paper also describes how to measure the enzymatic activity in cultured epimastigotes, infected Vero cells with amastigotes, and trypomastigotes released from the cultured cells using the reference drug, benznidazole, as an example. This colorimetric assay is easily performed and can be scaled to a high-throughput format and applied to other T. cruzi strains.
Introduction
Chagas disease (ChD), or American trypanosomiasis, is a parasitic disease caused by the flagellated protozoan, Trypanosoma cruzi (T. cruzi). ChD begins with an asymptomatic or oligosymptomatic acute phase that is usually undiagnosed, followed by a lifelong chronic phase. In the chronicity, ~30% of patients manifest-decades after the infection-a variety of debilitating conditions, including myocardiopathy, mega-digestive syndromes, or both, with a mortality rate ranging from 0.2% to 20%1,2,3. Asymptomatic chronic patients may have no clinical signs but remain seropositive throughout their life.
Estimations suggest that ~7 million people are infected worldwide, mostly from Latin America, where ChD is endemic. In these countries, T. cruzi is mainly transmitted through infected blood-sucking triatomine bugs (vector-borne transmission) and less frequently by oral transmission through the ingestion of food contaminated with triatomine feces containing the parasites2. Additionally, the parasite can be transmitted via the placenta from chagasic mothers to newborns, through blood transfusions, or during organ transplantation. These vector-independent ways of acquiring the infection and human migration have contributed to the worldwide spread of the disease, evidenced by an increasing number of cases in North America, Europe, and some African, Eastern Mediterranean, and Western Pacific countries4. ChD is considered a neglected disease as vector-borne transmission is closely associated with poverty and is a leading public health issue, especially in Latin American low-income countries. Although there are available treatments, mortality due to ChD in Latin America is the highest among parasitic diseases, including malaria2.
There are two registered drugs for ChD treatment introduced in the late 1960s and early 1970s: nifurtimox and benznidazole5. Both drugs are effective in the acute phase of the disease in adults, children, and congenitally infected newborns, as well as in children with chronic infection, where cure is usually achieved. However, only a few people are diagnosed early enough to be treated in time. According to the latest clinical trials, both drugs have important limitations in adults and were ineffective in reducing symptoms in people with chronic disease; hence, their use in this stage is controversial. Other drawbacks are the prolonged treatment periods required (60-90 days) and the frequent, severe adverse effects observed, which lead to discontinuation of therapy in a proportion of infected people6,7. It is estimated that fewer than 10% of the people with ChD have been diagnosed, and even fewer have access to treatment, as many affected individuals live in rural areas with no or scarce access to healthcare8. These facts highlight the urgent need to find new drugs against T. cruzi to allow for more efficient, safe, and applicable-to-the-field treatments, especially for the chronic phase. In this regard, another challenge in the development of more efficacious compounds is the limitation of systems for assessing drug efficacy in vitro and in vivo9.
Although chemical biology and genomic approaches for the identification of potential drug targets have been used in kinetoplastid parasites, the available genomic tools in T. cruzi are limited in contrast to T. brucei or Leishmania. Thus, the screening of compounds with trypanocidal activity is still the most used approach in the search for new chemotherapeutic drug candidates against ChD. Usually, drug discovery in T. cruzi must start with testing the effects of a new drug in an in vitro assay against the epimastigote stage. For decades, the only way for measuring the inhibitory effects of candidate compounds on T. cruzi was manual microscopic counting, which is laborious, time-consuming, and operator-dependent. Moreover, this approach is suitable for assaying a small number of compounds but is unacceptable for high-throughput screening of large compound libraries. Nowadays, many investigations begin with the analysis of a vast number of compounds from different origins that are assayed in vitro, testing their capacity for inhibiting parasite growth. Both colorimetric and fluorometric methods have been developed to increase throughput in these assays, improving the objectivity of the screening and making the whole process less tedious9.
One of the most widely used colorimetric methods is based on the β-galactosidase activity of transfected parasites first described by Bucknet and collaborators10. The β-galactosidase enzyme expressed by the recombinant parasites hydrolyzes the chromogenic substrate, chlorophenol red β-D-galactopyranoside (CPRG), to chlorophenol red, which can be easily measured colorimetrically using a microplate spectrophotometer. Thus, parasite growth in the presence of a variety of compounds can be simultaneously evaluated and quantitated in microtiter plates. This method has been applied to test drugs in epimastigote forms (present in the insect vector), trypomastigotes, and intracellular amastigotes, the mammalian stages of the parasite. Further, several recombinant T. cruzi strains transfected with the pBS:CL-Neo-01/BC-X-10 plasmid (pLacZ)10 to express the Escherichia coli β-galactosidase enzyme are already available (and new ones can be constructed), which allows the evaluation of parasites from different discrete typing units (DTUs) that may not behave equally toward the same compounds10,11,12,13. This method has already been successfully used to evaluate compounds for activity against T. cruzi in low- and high-throughput screening12,13. Similar approaches have also been used in other protozoan parasites, including Toxoplasma gondii and Leishmania mexicana14,15.
This paper describes and shows a detailed method for an in vitro drug screening against all life cycle stages of T. cruzi using parasites expressing β-galactosidase. The assays presented here have been performed with a β-galactosidase-expressing T. cruzi line obtained by transfection of T. cruzi Dm28c strain from DTU I13 with pLacZ plasmid (Dm28c/pLacZ). Additionally, the same protocol could be easily adapted to other strains to compare the performance between compounds and between T. cruzi strains or DTUs.
Protocol
NOTE: An overview of the entire experimental design is depicted in Figure 1.
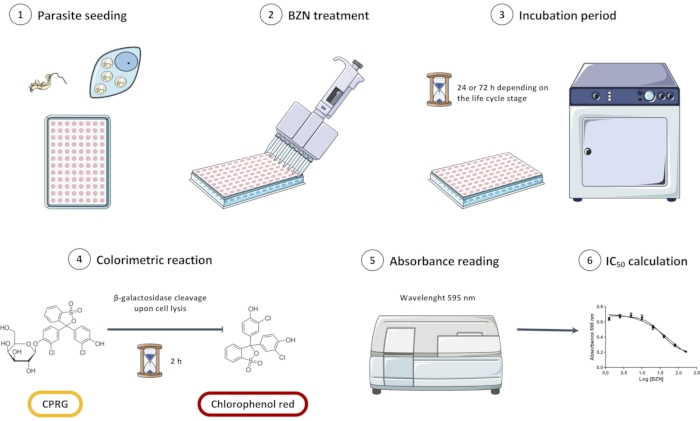
Figure 1: Overview of the in vitro screening assay of Trypanosoma cruzi Dm28c/pLacZ line using CPRG as a substrate for the colorimetric reaction. The assay consists of seeding the parasites (1), incubating them with BZN (2 and 3), and then adding the colorimetric substrate (4). When parasites are lysed, β-galactosidase is released and cleaves CPRG to chlorophenol red; this change in color can be measured spectrophotometrically (5). Data can be analyzed in statistical analysis software to obtain the half inhibitory concentration (IC50) of BZN. Abbreviations: CPRG = chlorophenol red β-D-galactopyranoside; BZN = benznidazole. Please click here to view a larger version of this figure.
1. Preparation of stock solutions
- Preparation of media and solutions
- Hemin solution (Supplemental Table S1)
- Add all the components to a 50 mL centrifuge tube in the order given in the recipe and homogenize by inversion several times.
- Sterilize by filtration through a 0.22 µm filter.
- Prepare 1 mL aliquots in 1.5 mL microcentrifuge tubes and keep them at -80 °C until use.
- Liver Infusion Tryptose (LIT) Medium (Supplemental Table S1)
- Weigh all the components and stir to homogenize at room temperature in a 1 L beaker containing at least 700 mL of distilled water.
- Adjust the pH to 7.2 and top up the volume to 900 mL in a 1 L graduated cylinder with distilled water; sterilize by filtration or autoclaving (121 °C for 20 min).
- Supplement the medium by adding 100 mL of fetal calf serum (FCS), (10% FCS, sterile and heat-inactivated at 56 °C for 45 min), 20 mL of 40% sterile glucose solution (sterilized by autoclaving, 121 °C for 20 min), and 5 mL of hemin solution (final concentration 5 µM) to 900 mL of LIT medium.
- Prepare Dulbecco's Modified Eagle Medium (DMEM) from the powder following the manufacturer's instructions.
- Phosphate-buffered saline (PBS) (Supplemental Table S1)
- Dissolve all solid components by stirring the solution at room temperature in a 1 L beaker.
- Adjust the pH to 7.2, level up to 1 L in a 1 L graduated cylinder with distilled water, and sterilize by filtration or autoclaving (121 °C, 20 min).
- Hemin solution (Supplemental Table S1)
- Benznidazole (BZN) stock solutions and dilutions
NOTE: The range of BZN concentration used in this work was 2.5 to 80 µM.- Prepare a stock solution of 1 M BZN by dissolving 13 mg of the drug in 50 µL of dimethylsulfoxide (DMSO). Under aseptic conditions, prepare serial dilutions from this 1 M BZN stock solution at twice the final desired concentration (2x solutions) in a final volume that is adequate for the number of wells to be assayed.
NOTE: Calculate for 100 µL per well with an excess of 10-20%. The BZN stock solution and all BZN dilutions must be prepared immediately before use in the assay due to the low solubility of the drug in the medium. - Prepare 2x BZN dilutions of 160, 80, 40, 20, 10, and 5 µM.
- Dilute 1 M BZN stock solution at a 100-fold dilution (10 µL of 1 M BZN + 990 µL of medium) to obtain a 10 mM solution in the appropriate medium used for each life cycle stage of T. cruzi. Mix continuously to homogenize the suspension.
- Dilute 10 mM BZN solution to prepare 320 µM BZN in the appropriate medium: 32 µL of 10 mM BZN + 968 µL of medium. Mix continuously to homogenize the suspension.
- Dilute 320 µM BZN 2-fold to obtain a concentration of 160 µM (500 µL of 320 µM BZN + 500 µL of medium). Mix continuously to homogenize the suspension. Repeat this 2-fold dilution with each resulting solution to obtain 80, 40, 20, 10, and 5 µM solutions.
- Dilute DMSO 1,000-fold in the appropriate medium for use as untreated control (100% survival control).
NOTE: Epimastigotes tolerate up to a 100-fold dilution of DMSO, whereas Vero cells tolerate only up to a 1,000-fold dilution of DMSO. If necessary, a death control with 50% DMSO can be included as the 0% survival condition.
- Prepare a stock solution of 1 M BZN by dissolving 13 mg of the drug in 50 µL of dimethylsulfoxide (DMSO). Under aseptic conditions, prepare serial dilutions from this 1 M BZN stock solution at twice the final desired concentration (2x solutions) in a final volume that is adequate for the number of wells to be assayed.
- Substrate solution
- Dissolve CPRG at 1 mM concentration in distilled water. For a 96-well plate, add 2.4 mg of CPRG to 4 mL of water.
NOTE: CPRG solution must be prepared immediately before the assay.
- Dissolve CPRG at 1 mM concentration in distilled water. For a 96-well plate, add 2.4 mg of CPRG to 4 mL of water.
- Lysis solution
- Prepare a 2.5% v/v solution of nonionic, non-denaturing detergent 2-[4-(2,4,4-trimethylpentan-2-yl)phenoxy]ethanol (see the Table of Materials) in 1x PBS. Prepare 1 mL of the solution per 96-well plate immediately before the assay.
2. Parasite culture preparation
- Epimastigote preparation
NOTE: T. cruzi Dm28c/pLacZ line13 is used throughout this report.- Grow the β-galactosidase-expressing T. cruzi epimastigotes axenically at 28 °C in cell culture flasks with a growth area of 25 cm2 (T-25 flasks). Maintain the cultures in log phase by subculturing every 48-72 h (in 5 mL) in LIT medium supplemented with 10% FCS (Supplemental Table S1) and geneticin sulfate (G418) at a final concentration of 200 µg/mL. Quantify parasite growth by cell counting in a Neubauer chamber before subculturing. Securely close the cap and keep the culture flask (not vented) at 28 °C in a vertical position.
NOTE: G418 ensures pLacZ plasmid selection and maintenance. Log phase cultures have an epimastigote concentration of 1-5 × 107 parasites/mL for the Dm28c/pLacZ line. - Prepare a suspension of 2 × 105 epimastigotes/mL from a log phase culture in LIT supplemented with G418 antibiotic. Dispense 100 µL of the epimastigote suspension per well (20,000 epimastigotes in 100 µL of LIT) of a 96-well microplate and make up the final volume to 200 µL per well with the medium.
- Grow the β-galactosidase-expressing T. cruzi epimastigotes axenically at 28 °C in cell culture flasks with a growth area of 25 cm2 (T-25 flasks). Maintain the cultures in log phase by subculturing every 48-72 h (in 5 mL) in LIT medium supplemented with 10% FCS (Supplemental Table S1) and geneticin sulfate (G418) at a final concentration of 200 µg/mL. Quantify parasite growth by cell counting in a Neubauer chamber before subculturing. Securely close the cap and keep the culture flask (not vented) at 28 °C in a vertical position.
- Amastigote preparation
- Use spontaneous metacyclic trypomastigotes obtained from an aged epimastigote culture (for 7 days in this protocol) to perform an initial infection in a T-25 flask with 2 × 105 Vero cells seeded previously in DMEM supplemented with 2% FCS.
- Count the number of metacyclic trypomastigotes in a Neubauer chamber, infect the Vero cell monolayer with a multiplicity of infection (MOI) of 10 in 5 mL of DMEM with 2% FCS, and incubate at 37 °C and 5% CO2 for 16 h. Wash the remaining trypomastigotes by removing the medium from the flask with a 5 mL sterile pipette, then add 5 mL of 1x PBS and aspirate. Finally, add 5 mL of DMEM with 2% FCS and incubate under the same conditions.
- Use the trypomastigotes emerging from the infected Vero cell monolayer to maintain the infection in T-25 flasks with 2 × 105 Vero cells in DMEM with 2% FCS, generating a new infected bottle every week.
NOTE: After 5-7 days, trypomastigotes start to emerge and are visible in the supernatant. Do not add G418 to the trypomastigotes used to infect the cells or the infected cells, as the Vero cell line is not resistant to G418.
- Prepare a suspension of 1 × 105 Vero cells/mL in DMEM supplemented with 2% FCS and seed 100 µL of the suspension per well in 96-well tissue culture plates (10,000 cells per well). Incubate overnight (12-16 h) at 37 °C and 5% CO2 to ensure cell adherence to the bottom of the wells.
- After the overnight incubation, rinse the Vero cell monolayer three times with 100 µL of sterile 1x PBS. Add T. cruzi Dm28c/pLacZ trypomastigotes (obtained from a previous infection in a T-25 flask, step 2.2.1) at an MOI of 10 in 100 µL of DMEM supplemented with 2% FCS per well (100,000 trypomastigotes per well).
- Incubate the plates for 6 h at 37 °C and 5% CO2. After this incubation period, wash the plates twice with 1x PBS, and add 100 µL of DMEM without phenol red supplemented with 2% FCS.
NOTE: After 48 h (2 days post infection), intracytoplasmic amastigotes are visible with an optic microscope. Phenol red, a pH indicator in DMEM and other cell culture media, could interfere with the absorbance measurement of CPRG. If DMEM without phenol red is not available, see alternatives mentioned below in section 3.2.1.
- Use spontaneous metacyclic trypomastigotes obtained from an aged epimastigote culture (for 7 days in this protocol) to perform an initial infection in a T-25 flask with 2 × 105 Vero cells seeded previously in DMEM supplemented with 2% FCS.
- Trypomastigote preparation
- Prepare a suspension of 1 × 106 Vero cells/mL in DMEM supplemented with 2% FCS and seed 800,000 cells in 5 mL of the medium in T-25 flasks. Incubate overnight (12-16 h) at 37 °C and 5% CO2 to ensure cell adherence.
NOTE: For a T-75 flask, seed 2 × 106 cells in a final volume of 15 mL. - After incubation, rinse two times with 3 mL of sterile 1x PBS. Add T. cruzi Dm28c/pLacZ trypomastigotes at an MOI of 10 in 5 mL of DMEM with 2% FCS (8 × 106 trypomastigotes for a T-25 flask).
NOTE: For a T-75 flask, add 20 × 106 trypomastigotes in a final volume of 15 mL of DMEM with 2% FCS. - Incubate overnight (12-16 h) at 37 °C and 5% CO2. Wash the flask twice with 3 mL of 1x PBS, and add 5 mL of fresh DMEM supplemented with 2% FCS. Incubate at 37 °C and 5% CO2 for four days.
- Check the supernatant for trypomastigotes under an optic microscope. Quantify the trypomastigotes by counting them in a Neubauer chamber. Collect the supernatant in a 15 mL tube and centrifuge at 7,000 × g for 10 min at room temperature.
- Discard the supernatant and resuspend the pellet to obtain a concentration of 1 × 106 trypomastigotes/mL in DMEM without phenol red supplemented with 2% FCS. Seed 100 µL of the trypomastigote suspension (100,000 trypomastigotes per well) in a 96-well plate.
NOTE: If DMEM without phenol red is not available, see alternatives below in section 3.2.1.
- Prepare a suspension of 1 × 106 Vero cells/mL in DMEM supplemented with 2% FCS and seed 800,000 cells in 5 mL of the medium in T-25 flasks. Incubate overnight (12-16 h) at 37 °C and 5% CO2 to ensure cell adherence.
3. β-galactosidase assay
NOTE: Quantitation of β-galactosidase activity is used as an indirect way of determining the number of parasites. It is expected that growth will be inhibited in the presence of a trypanocidal compound, leading to a lower number of parasites compared to the untreated control, which will be reflected in a lower β-galactosidase activity and therefore lower absorbance.
- Incubate the parasites with BZN.
- Add 100 µL of corresponding 2x BZN solution per well to reach a final concentration of BZN of 80, 40, 20, 10, 5, and 2.5 µM to 100 µL of epimastigote suspension (from step 2.1), Vero cells with amastigotes (2 days post infection) (step 2.2), or trypomastigotes (step 2.3) in a 96-well plate.
- Incubate the epimastigotes at 28 °C for 72 h, and the trypomastigotes or infected Vero cells with amastigotes for 24 h at 37 °C and 5% CO2.
NOTE: Each drug concentration should be evaluated at least in triplicate and include control cultures of epimastigotes, trypomastigotes, and infected Vero cells with DMSO (see step 1.2.2.4).
- Colorimetric reaction
- After the treatment incubation period, if infected Vero cells or trypomastigotes are in DMEM with phenol red, replace the medium with 100 µL of 1x PBS to avoid interference. Perform triplicate blank wells containing only 100 µL of corresponding medium (or 1x PBS as appropriate).
NOTE: It is not necessary to remove the culture medium for epimastigotes in case of LIT medium or DMEM without phenol red. DMEM with phenol red can still be used; prepare a blank well with DMEM alone to measure the base absorbance and then subtract this value during data analysis (step 3.3.). Schneider's insect medium, which is colorless, is an alternative for epimastigotes. - Add 40 µL of CPRG substrate solution and 10 µL of the detergent solution to each well, obtaining a final concentration of 200 µM CPRG and 0.1% detergent in a final volume of 250 µL in each well.
NOTE: The CPRG solution and detergent can be added together in a final volume of 50 µL per well. - Incubate at 37 °C for 2 h and measure the absorbance at 595 nm in a microplate spectrophotometer.
NOTE: The expected color change is yellow to reddish-brown upon β-galactosidase enzymatic cleavage (Figure 2A). Incubation time can be extended up to 4 h, and the absorbance spectra of chlorophenol red can be read between 570 and 595 nm with similar curve fittings (Supplemental Figure S1A,B). Incubation for up to 24 h in the presence of CPRG substrate has shown similar curve fittings (Supplemental Figure S1C).- In a microplate spectrophotometer with a monochromator selector, create a new protocol in the equipment software (Supplemental Figure S2).
- Click Absorbance as detection method | Endpoint as read type | Ok (Supplemental Figure S2A). Add a Read Step, type the selected wavelength, and click Ok (Supplemental Figure S2B).
- In the Plate Layout section, mark the wells to be read and click Ok (Supplemental Figure S2C). To read the plate, insert it in the tray and click on Read Plate. Wait for the values to appear on the screen (Supplemental Figure S2D) and export them to a spreadsheet to analyze the results.
- After the treatment incubation period, if infected Vero cells or trypomastigotes are in DMEM with phenol red, replace the medium with 100 µL of 1x PBS to avoid interference. Perform triplicate blank wells containing only 100 µL of corresponding medium (or 1x PBS as appropriate).
- Data analysis and media inhibitor concentration (IC50) calculation
- Subtract the blank measured value, corresponding to only LIT medium, 1x PBS, or DMEM with or without phenol red plus the CPRG-detergent solution. When testing the trypanocidal activity of colored compounds, measure the absorbance of additional blank controls with LIT or DMEM with each concentration of drug used and then subtract those values from the absorbance values obtained with the parasites at each concentration.
NOTE: Supplemental Table S2 shows typical values obtained in this assay with these media without parasites plus CPRG-detergent solution. The differences before and after adding CPRG are significant but do not interfere with the assay with parasites (Supplemental Table S2). - In statistical analysis software, plot the concentration of BZN (in µM) versus the absorbance at 595 nm in an xy table. Transform the BZN concentrations to logarithmic values by clicking on the Analyze button, selecting the Transform option | transform the x-values using x=log(x) option, and clicking the Ok button.
- Obtain the IC50 values from the statistical analysis software.
NOTE: The IC50 is defined as the drug concentration that reduces parasite growth by 50% compared to the untreated control and is calculated as the inflection point of the sigmoidal function that fits the curve.- In the statistical analysis software, click on the Analyze button, select Non-linear regression (curve fit) in the xy analysis list, and click Ok.
- In the model tab of the Parameters window, in the dose-response - inhibition group of built-in equations, select the option dose-response method: log(inhibitor) vs. response - Variable Slope (four parameters). Leave all the other tabs at default values; click Ok.
- Click on the results section of the statistical analysis software to find the IC50 value, the SD, and the goodness of the fit.
- Click on the graph section to find the xy graph of the logarithmic concentration of the drug versus the absorbance values. Look for the curve fit is also graphed in a different color.
NOTE: A free online IC50 calculation tool can be found at https://www.aatbio.com/tools/ic50-calculator.
- Subtract the blank measured value, corresponding to only LIT medium, 1x PBS, or DMEM with or without phenol red plus the CPRG-detergent solution. When testing the trypanocidal activity of colored compounds, measure the absorbance of additional blank controls with LIT or DMEM with each concentration of drug used and then subtract those values from the absorbance values obtained with the parasites at each concentration.
Results
Following the protocol described above, β-galactosidase-expressing Dm28c epimastigotes were incubated with 6 concentrations of BZN (2.5, 5, 10, 20, 40, 80 µM) (or compounds of interest) for 72 h. After this period, CPRG reagent was added along with detergent, which lyses the cells and releases β-galactosidase. CPRG is cleaved by the β-galactosidase to produce chlorophenol red, leading to a change in color from yellow to reddish (Figure 2A). Chlorophenol red was measured b...
Discussion
This paper describes an assay based on determining the cytoplasmic β-galactosidase activity released due to membrane lysis of T. cruzi epimastigotes, trypomastigotes, or infected cells with amastigotes in the presence of the substrate CPRG. We used T. cruzi Dm28c/pLacZ parasites, a stable parasite strain obtained after transfection with a β-galactosidase-bearing plasmid constructed by Buckner and co-authors10. This assay has been used to search for antitrypanocidal comp...
Disclosures
The authors have no conflict of interest to disclose.
Acknowledgements
We thank Dr. Buckner for kindly providing the pLacZ plasmid. This work was supported by Agencia Nacional de Promoción Científica y Tecnológica, Ministerio de Ciencia e Innovación Productiva from Argentina (PICT2016-0439, PICT2019-0526, PICT2019-4212), and Research Council United Kingdom [MR/P027989/1]. Servier Medical Art was used to produce Figure 1 (https://smart.servier.com).
Materials
| Name | Company | Catalog Number | Comments |
| 1 L beaker | Schott Duran | 10005227 | |
| 10 mL serological pipette sterile | Jet Biofil | GSP211010 | |
| 5 mL serological pipette sterile | Jet Biofil | GSP010005 | |
| 96-well plates | Corning | 3599 | |
| Benznidazole | Sigma Aldrich | 419656 | N-Benzyl-2-nitro-1H-imidazole-1-acetamide |
| Biosafty Cabinet | Telstar | Bio II A/P | |
| Centrifuge tube 15 mL conical bottom sterile | Tarson | 546021 | |
| Centrifuge tube 50 mL conical bottom sterile | Tarson | 546041 | |
| CO2 Incubator | Sanyo | MCO-15A | |
| CPRG | Roche | 10 884308001 | Chlorophenol Red-β-D-galactopyranoside |
| DMEM, High Glucose | Thermo Fisher Cientific | 12100046 | Powder |
| DMSO | Sintorgan | SIN-061 | Dimethylsulfoxid |
| Fetal Calf Serum | Internegocios SA | FCS FRA 500 | Sterile and heat-inactivated |
| G418 disulphate salt solution | Roche | G418-RO | stock concentration: 50 mg/mL |
| Glucose D(+) | Cicarelli | 716214 | |
| Graduated cylinder | Nalgene | 3663-1000 | |
| Hemin | Frontier Scientific | H651-9 | |
| KCl | Cicarelli | 867212 | |
| Liver Infusion | Difco | 226920 | |
| Microcentrifuge tube 1.5 mL | Tarson | 500010-N | |
| Microplate Spectrophotometer | Biotek | Synergy HTX | |
| Na2HPO4 | Cicarelli | 834214 | |
| NaCl | Cicarelli | 750214 | |
| Neubauer chamber | Boeco | BOE 01 | |
| Nonidet P-40 | Antrace | NIDP40 | 2-[4-(2,4,4-trimethylpentan-2-yl)phenoxy]ethanol |
| Prism | Graphpad | Statistical Analysis software | |
| Sodium Bicarbonate | Cicarelli | 929211 | NaHCO3 |
| Sorvall ST 16 Centrifuge | Thermo Fisher Cientific | 75004380 | |
| T-25 flasks | Corning | 430639 | |
| Tryptose | Merck | 1106760500 | |
| Vero cells | ATCC | CRL-1587 |
References
- Rassi, A., Rassi, A., Rassi, S. G. Predictors of mortality in chronic Chagas disease: a systematic review of observational studies. Circulation. 115 (9), 1101-1108 (2007).
- Pérez-Molina, J. A., Molina, I. Chagas disease. The Lancet. 391 (10115), 82-94 (2018).
- Messenger, L. A., Miles, M. A., Bern, C. Between a bug and a hard place: Trypanosoma cruzi genetic diversity and the clinical outcomes of Chagas disease. Expert Review of Anti-infective Therapy. 13 (8), 995-1029 (2015).
- Steverding, D. The history of Chagas disease. Parasites & Vectors. 7, 317 (2014).
- Viotti, R., et al. Towards a paradigm shift in the treatment of chronic Chagas disease. Antimicrobial Agents and Chemotherapy. 58 (2), 635-639 (2014).
- Bern, C. Chagas’ Disease. The New England Journal of Medicine. 373 (19), 1882 (2015).
- Bustamante, J. M., Tarleton, R. L. Methodological advances in drug discovery for Chagas disease. Expert Opinion on Drug Discovery. 6 (6), 653-661 (2011).
- Buckner, F. S., Verlinde, C. L., La Flamme, A. C., Van Voorhis, W. C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrobial Agents and Chemotherapy. 40 (11), 2592-2597 (1996).
- Vega, C., Rolón, M., Martínez-Fernández, A. R., Escario, J. A., Gómez-Barrio, A. A new pharmacological screening assay with Trypanosoma cruzi epimastigotes expressing beta-galactosidase. Parasitology Research. 95 (4), 296-298 (2005).
- Bettiol, E., et al. Identification of three classes of heteroaromatic compounds with activity against intracellular Trypanosoma cruzi by chemical library screening. PLoS Neglected Tropical Diseases. 3 (2), 384 (2009).
- Gulin, J. E. N., et al. Optimization and biological validation of an in vitro assay using the transfected Dm28c/pLacZ Trypanosoma cruzi strain. Biology Methods and Protocols. 6 (1), 004 (2021).
- da Silva Santos, A. C., Moura, D. M. N., Dos Santos, T. A. R., de Melo Neto, O. P., Pereira, V. R. A. Assessment of Leishmania cell lines expressing high levels of beta-galactosidase as alternative tools for the evaluation of anti-leishmanial drug activity. Journal of Microbiological Methods. 166, 105732 (2019).
- McFadden, D. C., Seeber, F., Boothroyd, J. C. Use of Toxoplasma gondii expressing beta-galactosidase for colorimetric assessment of drug activity in vitro. Antimicrobial Agents and Chemotherapy. 41 (9), 1849-1853 (1997).
- Moreno-Viguri, E., et al. In vitro and in vivo anti-Trypanosoma cruzi activity of new arylamine Mannich base-type derivatives. Journal of Medicinal Chemistry. 59 (24), 10929-10945 (2016).
- García, P., Alonso, V. L., Serra, E., Escalante, A. M., Furlan, R. L. E. Discovery of a biologically active bromodomain inhibitor by target-directed dynamic combinatorial chemistry. ACS Medicinal Chemistry Letters. 9 (10), 1002-1006 (2018).
- Vela, A., et al. In vitro susceptibility of Trypanosoma cruzi discrete typing units (DTUs) to benznidazole: A systematic review and meta-analysis. PLoS Neglected Tropical Diseases. 15 (3), 0009269 (2021).
- Alonso-Padilla, J., Rodríguez, A. High throughput screening for anti-Trypanosoma cruzi drug discovery. PLoS Neglected Tropical Diseases. 8 (12), 3259 (2014).
- Martinez-Peinado, N., et al. Amaryllidaceae alkaloids with anti-Trypanosoma cruzi activity. Parasites & Vectors. 13 (1), 299 (2020).
- Puente, V., Demaria, A., Frank, F. M., Batlle, A., Lombardo, M. E. Anti-parasitic effect of vitamin C alone and in combination with benznidazole against Trypanosoma cruzi. PLoS Neglected Tropical Diseases. 12 (9), 0006764 (2018).
- Muelas-Serrano, S., Nogal-Ruiz, J. J., Gómez-Barrio, A. Setting of a colorimetric method to determine the viability of Trypanosoma cruzi epimastigotes. Parasitology Research. 86 (12), 999-1002 (2000).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved