Method Article
A High-Throughput Multiplexed Screening for Type 1 Diabetes, Celiac Diseases, and COVID-19
In This Article
Summary
In line with the urgent need for screening for type 1 diabetes, celiac disease, and coronavirus disease 2019, we developed a high throughput 6-Plex electrochemiluminescence assay to simultaneously detect all four islet autoantibodies, tissue transglutaminase autoantibodies, and antibodies to the receptor binding domain of severe acute respiratory syndrome coronavirus 2.
Abstract
An ongoing clinical trial, Autoimmunity Screening for Kids (ASK), is the first screening study in the general population for type 1 diabetes (T1D) and celiac disease in the United States. With the coronavirus disease 2019 (COVID-19) pandemic, the epidemiology of COVID-19 in the general population and knowledge about the association between COVID-19 infection and T1D development are urgently needed. The currently standard screening method of the radio-binding assay (RBA) has met two great challenges: low efficiency with a single assay format and low disease specificity with a large proportion of low-affinity antibodies generated in screening. With the platform of the multiplex electrochemiluminescence (ECL) assay we established previously, a novel 6-Plex ECL assay was developed that combines, in a single well, all four islet autoantibodies (IAbs) to insulin, glutamic acid decarboxylase (GAD65), insulinoma antigen 2 (IA-2), and Zinc transporter 8 (ZnT8) for T1D, transglutaminase autoantibodies (TGA) for celiac disease, and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) receptor-binding domain (RBD) antibodies for COVID-19. The assay was validated in blind using 880 samples from the ASK study, including 325 positive samples and 555 all antibody-negative samples, and compared with the standard RBAs and a single ECL assay. With the advantages of high efficiency, low cost, and low serum volume, this assay has been accepted as the primary screening tool for the ASK study.
Introduction
Large epidemiologic studies worldwide demonstrate that the incidence of type 1 diabetes (T1D) has been rapidly increasing by 3%-5% annually, and the prevalence of T1D has doubled in the last 20 years, especially in young children1,2. Islet autoantibodies (IAbs) usually appear years before clinical symptoms and are currently the most reliable predictive and diagnostic biomarkers for T1D3. Screening for T1D autoantibodies can identify people at risk for progressing to clinical T1D, educate the public, largely reduce the life-threatening complication of ketoacidosis, and benefit clinical trials for prevention therapies. Worldwide prevention efforts for T1D are underway and multiple interventional trials are being carried out among subjects with IAbs positive to delay the progression to clinical T1D, and the Barbara Davis Center for Diabetes launched the first US clinical trial of screening in the general population in 2016 for T1D and celiac disease, Autoimmunity Screening for the Kids (ASK)4. Celiac disease (CD) is a chronic intestinal inflammatory disease related to immune and genetic factors. The prevalence of CD in children with T1D can reach up to 24.5%5. More than half of individuals with CD may not have typical symptoms at presentation6, so serological screening for celiac disease is quite necessary.
Since the pandemic of coronavirus disease 2019 (COVID-19) started, over 200 million cases have been reported globally, and children account for up to 16% of laboratory-confirmed cases7. Many studies have demonstrated the impact of existing T1D on the severity and fatality of COVID-19 infection8, while the impact of COVID-19 infection on T1D development is not clear. The investigation of the total rate of COVID-19 infection in the general population by the determination of severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) antibodies and the study of the association between COVID-19 infection and triggering T1D autoimmunity or accelerating T1D progression are urgently needed and very important, while the ongoing ASK study is an excellent platform for this. Due to the single assay format and generation of a large proportion of low-affinity antibody positivity, the standard radio-binding assay (RBA) has met a bottleneck of low efficiency and low disease specificity9. In the present paper, we present a novel 6-Plexed electrochemiluminescence (ECL) assay built on our previous multiplex ECL assay platform using a multiple-Plex plate, combining six autoantibody assays in one single well, including all four major IAbs to insulin (IAA), glutamic acid decarboxylase-65 (GADA), insulinoma antigen-2 (IA-2A), and Zinc transport-8 (ZnT8A), autoantibodies to transglutaminase (TGA) for celiac disease, and SARS-CoV-2 receptor-binding domain (RBD) antibodies (COVID-19A). This is the first multiplex assay that recruited a complete panel of four major IAbs and further combined this with TGA and COVID-19A. It has been validated and formally accepted as the primary screening tool replacing the standard RBA in the ASK study.
Protocol
The research protocol was approved by the Colorado Multiple Institutional Review Board.
1. Buffer Preparation
- Prepare the labeling buffer (2x PBS, pH 7.9): Add 100 mL of 10x PBS to 400 mL of distilled water and adjust the pH using NaOH to 7.9.
- Make 3 mM of biotin and Ru Sulfo-NHS: Dissolve 1 mg of biotin in 588 µL of labeling buffer and dissolve 150 nmol of Ru Sulfo-NHS in 50 µL of labeling buffer.
- Prepare the antigen buffer (1% BSA): Dissolve 5 g of bovine serum albumin (BSA) in 500 mL of 1x PBS.
- Prepare the coating buffer (3% Blocker A): Dissolve 15 g of Blocker A in 500 mL of 1x PBS.
- Prepare the first washing buffer (0.05 % Tween 20, PBST): Mix 2.5 mL of Tween 20 in 5,000 mL of 1x PBS.
- Prepare the second washing buffer (0.4 M NaCl): Mix 50 mL of 5 M NaCl in 575 mL of distilled water to make the 0.4 M NaCl solution.
NOTE: For efficient labeling, always use freshly prepared biotin and Ru Sulfo-NHS solutions and not stored ones made previously.
2. Antigen protein labeling with biotin and Ru Sulfo-NHS separately
NOTE: A higher concentration of antigen protein ≥0.5 mg/mL is recommended for higher labeling efficiency.
- Prepare six targeted antigen protein solutions, including proinsulin, GAD-65, IA-2, ZnT8, TG, and receptor-binding domain (RBD) of the spike protein of SARS-CoV-2. Calculate the moles of each antigen protein according to its molecular weight and make appropriate molar ratios of biotin or Ru Sulfo-NHS. The ratio of antigen to the sum of biotin or Ru Sulfo-NHS will be 1:5 for small molecular antigens (molecular weight ≤10 kd), such as proinsulin protein. For medium molecular antigens (molecular weight 10-50 kd), such as ZnT8, the ratio will adjust to 1:10. For large molecular weight antigens (molecular weight >50 kd), such as GAD, the molar ratio will be 1:20.
- Divide the antigen protein into two equal parts and separately mix it with biotin and Ru Sulfo-NHS using the calculated molar ratio in step 2.1. Incubate the mixture at room temperature (RT) for 1.5 h, avoiding light.
NOTE: All reducing chemicals such as the Tris or glycine in the buffer of antigen protein need to be removed by a sizing spin column primed with a buffer of 2x PBS (pH 7.9). - Prime the spin columns with 2x PBS (the size of the spin column, 2 mL or 5 mL, depends on the volume of the labeling antigen protein): Add 1 ml of 2x PBS buffer to the spin column, centrifuge it at 1,000 x g for 2 min, and repeat the step 2x more.
- Let the antigen protein-biotin or -Ru Sulfo-NHS mixture go through the primed spin column 1x (centrifuge the column at 1,000 x g for 2 min) to purification and stop the labeling reaction.
- Measure the final volume of the labeled antigen protein eluted from the spin columns, calculating the concentrations of biotin- or Ru Sulfo-NHS-labeled antigen protein. Make smaller aliquots of labeled antigen protein (50 µL/tube) and store them at −80 °C for long-term use.
NOTE: The coverage of protein after each spin column will be an approximate 90%-95% retention rate.
3. Define the best concentration and ratios for biotin- and Ru Sulfo-NHS-labeled antigens for the 6-Plex ECL assay (checkerboard assay)
NOTE: The checkerboard assay for each antigen is necessary before integration into the multiplexed assay.
- Apply the checkerboard assay for each antigen separately.
NOTE: Steps 3.2.-3.7. will use TGA as a brief example. The TGA checkerboard assay process is to simulate the assay process but with only one kind of antigen. - Make the serial dilution of the biotinylated tTG and Ru Sulfo-NHS-labeled tTG. The recommended working concentrations of the first mixture solution start from 240 ng/mL for both biotinylated and Ru Sulfo-NHS-labeled tTG. Assume the concentrations of both original biotinylated and Ru Sulfo-NHS-labeled tTG are 1.0 µg/µL. Make a 10x dilution of the original Ru Sulfo-NHS-labeled tTG and a 5x dilution of the original biotinylated tTG with 5% BSA.
- Mix 4 µL of biotinylated tTG protein with 156 µL of 5% BSA (antigen buffer) and 240 µL of the streptavidin-conjugated linker in one tube and incubate the solution at RT for 30 min. Then, add 160 µL of stop solution, and incubate at RT for another 30 min. Take 400 µL of the mixture and mix with 2 mL of stop solution. The concentration of the biotinylated tTG in the mixture is 240 ng/mL.
- To make a serial dilution for biotin-labeled ZnT8 antigen, prepare another five new tubes, take 1 mL of an aliquot from the mixture in step 3.3. and mix with 1 mL of stop solution in a new tube, and get the mixture with a concentration of 120 ng/mL. Repeat this step, make serial 1:1 dilutions, and get the biotin-labeled tTG at 60 ng/mL, 30 ng/mL, 15 ng/mL, and 7.5 ng/mL.
- Add 4 µL of Ru Sulfo-NHS-labeled tTG with 1.6 mL of stop solution, and get a mixture of Ru Sulfo-NHS-labeled tTG at a concentration of 240 ng/mL. With the same steps of 3.4., make serial 1:1 dilutions and get the Ru Sulfo-NHS-labeled ZnT8 antigen at 60 ng/mL, 30 ng/mL, 15 ng/mL, 7.5 ng/mL, and 3.75 ng/mL. The last concentration is 0 ng/mL, with only stop solution and no Ru Sulfo-NHS-labeled antigen.
- Prepare tTG positive control and negative control serum samples (pre-qualified and standardized by a single ECL tTG assay) and a 96-well PCR plate. Aliquot the positive and negative control serum samples to the left half and right half of the plate, respectively, with 7 µL in each well. Add 1x PBS to the plate, with 33 µL per well.
- On the left half of the PCR plate, add 24 µL of the serial diluted biotin-labeled tTG-linker solution to each well, one column with one concentration in order of highest to lowest. In the same way, add 16 µL of the serial diluted Ru Sulfo-NHS labeled tTG antigen to each well, one row with one concentration, and then thoroughly mix. Repeat the step on the right half of the PCR plate.
- Continue the rest of the assay steps described in steps 5.3.-9.1. until the raw counting data is obtained.
- Calculate the ratios of positive control signals to corresponding negative control signals. Determine the best concentration for the biotin-labeled antigen and the Ru Sulfo-NHS-labeled antigen with higher ratio values and lower background for the negative control sample. The optimal working concentrations of biotin and Ru Sulfo-NHS labeled antigen protein are shown below: 16.0 ng/mL and 8.0 ng/mL for GAD65, 18.8 ng/mL and 9.4 ng/mL for SARS-CoV-2 RBD, 42.0 ng/mL and 42.0 ng/mL for IA-2, 60.0 ng/mL and 60.0 ng/mL for tTG, 4.2 ng/mL and 4.2 ng/mL for ZnT8, and 31.3 ng/mL and 31.3 ng/mL for proinsulin.
4. Create linker-coupled antigen solution
- Dilute the biotin- and Ru Sulfo-NHS-labeled antigens with 5% BSA to the optimal working concentrations according to step 3.9.
- Bind pre-selected linkers to each biotinylated antigen protein. For one 96-well plate assay, add 4 µL of biotinylated GAD65, SARS-CoV-2 RBD, IA-2, tTG, ZnT8, and proinsulin antigen protein into a separate tube containing 156 µL of 1% BSA and mix with 240 µL of corresponding streptavidin-conjugated linkers 1, 2, 3, 8, 9, and 10 (Figure 1). Incubate the mixture for 30 min at RT.
- Aliquot 160 µL of stop solution in each tube and incubate the mixture at RT for 30 min. Take out 400 µL of the mixture solution from each tube and combine them together.
- Add 4 µL of Ru Sulfo-NHS labeled GAD65, SARS-CoV-2 RBD, IA-2, tTG, ZnT8, and proinsulin antigen to the mixture above, and then add 1.6 mL of stop solution and 3.2 mL of 1x PBS and mix. Now the antigen solution is ready for use in the assay.
5. Incubate serum samples with the labeled antigen
- Aliquot 7 µL of serum per well for a 96-well PCR plate and cover with sealing film. Preheat the sera at 56 °C for 30 min on a PCR machine. Briefly centrifuge the plate at 100 x g for 1 min.
- Add 63 µL of antigen solution (prepared in step 4.4) per well, and mix with the sera. Cover the PCR plate with sealing foil to avoid light.
- Shake the plate on a shaker at 450 rpm at RT for 2 h and then incubate the plate at 4 °C for 18-24 h.
6. Prepare the 6-Plex plate
- Take a 6-Plex plate from the 4 °C refrigerator, allowing the plate to come to RT. Add 150 µL of 3% Blocker A to each well of the 6-Plex plate.
- Put the plate back in 4 °C refrigerator, cover the plate with foil avoiding light, and incubate overnight.
NOTE: Assay steps in Day 1 include sections 4.-6.
7. Transfer serum/antigen incubates into the 6-Plex plate
- The next day, take the incubating 6-Plex plate from the refrigerator and dump all the buffer out of the plate. Pat the plate on paper towels to dry out.
- Wash the 6-Plex plate 3x with 150 µL of washing buffer per well each time. Dry the plate on paper towels, and aliquot serum/antigen incubates from each well of the overnight incubating PCR plate into two wells of the 6-Plex plate (30 µL per well for duplicates).
- Cover the plate with foil, then put the plate on a plate shaker, and shake with a speed of 450 rpm at RT for 1 h.
8. Wash the 6-Plex plate and add reading buffer
- Dump the solution in the 6-Plex plate and wash the plate 3x with 150 µL of 0.4 M NaCl washing buffer per well each time.
- After the third wash is complete, dry the plate against the paper towel and add 150 µL of reading buffer into each well.
NOTE: Air bubbles in the well affect the accuracy of the plate reader machine and should be avoided at all costs.
9. Read the plate and analyze the data
- Count the plate on an electrochemiluminescence analyzer. The reading results will be presented with the values in counts per second (CPS). Calculate the relative antibody index of each sample using the raw CPS obtained from the reading machine against the CPS of the internal standard positive and negative controls of each specific antibody (Index value = [CPS (sample) - CPS (negative standard)] / [CPS (positive standard) - CPS (negative standard)]).
- Determine negative or positive antibody results using the cutoff values that have been established at the 99.5th to 99.8th percentiles of 555 negative control samples from the ASK study (all antibodies negative samples without a history or family history of diabetes or any other type of autoimmune disease).
NOTE: Assay steps in Day 2 includes sections 7-9.
Results
Table 1, Table 2, and Table 3 illustrate the representative results. The raw CPS obtained from the reading machine are illustrated in Table 1. In Table 2, raw CPS were arranged and sorted by the six linkers and corresponding antibodies. Table 3 shows the calculated index values with CPS as described in the assay protocol. All raw counting values should be checked to avoid wrong final index results caused by bad duplicates. In Table 1, examples of bad duplicates are given, which resulted in an error for the final index calculation in Table 3.
This assay was validated in a blind manner using 880 selected samples from the ASK study with 325 IAbs positive samples and 555 all Abs negative samples. The levels of autoantibodies from 6-Plex ECL assay for the 880 samples were compared point-by-point with the levels of corresponding single ECL assays (Figure 2) and with corresponding single standard RBA (Figure 3). The cutoff, sensitivity, and specificity for each antibody are listed in Table 4. The sensitivity and specificity of this ECL-COVID-19A assay have been identified as 100% and 99.9%, respectively10.
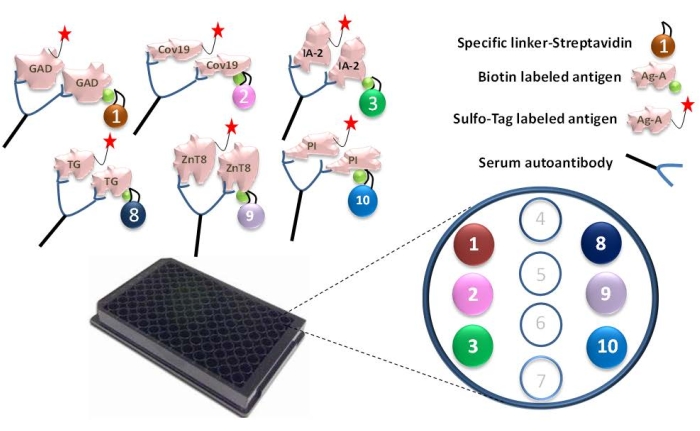
Figure 1: Illustration of the 6-Plex ECL assay. Serum autoantibodies make the connection of the Ru Sulfo-NHS-labeled antigen to the biotinylated antigen, which is coupled with a specific linker and forms a complex of antigen-antibody-antigen-linker. The complexes are captured through each specific linker onto the specific spots in the 6-Plex plate. For each antigen, the specific linker numbers were assigned: GAD-linker-1, Covid-19-linker-2, IA-2-linker-3, tTG-linker-8, ZnT8-linker-9, and Proinslulin-linker-10. This figure has been modified with permission from He et al.11. Please click here to view a larger version of this figure.
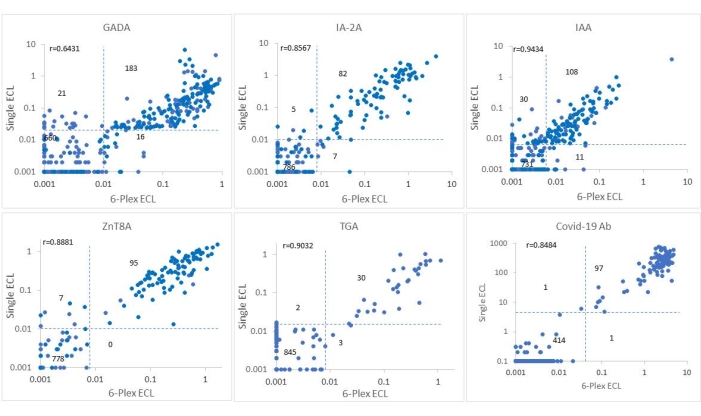
Figure 2: Comparison of six antibody levels in 880 samples from the ASK study between the single ECL assay and the 6-Plex ECL assay. The panels display comparisons of antibody levels for GADA, IAA, IA-2A, ZnT8A, TGA, and COVID-19A, respectively (index of COVID-19A was presented as (100 x calculated index value)). The dotted lines represent the assay cutoffs for each antibody assay. This figure has been modified with permission from He et al.11. Please click here to view a larger version of this figure.
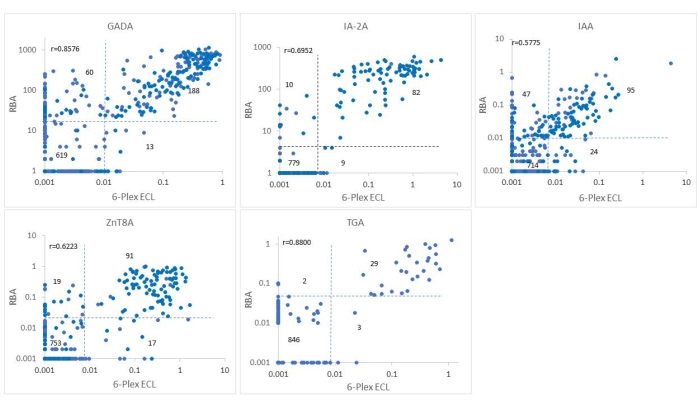
Figure 3: Comparison of five antibody levels in 880 samples from the ASK study between the RBA and the 6-Plex ECL assay. The panels display comparisons of antibody levels for GADA, IAA, IA-2A, ZnT8A, and TGA, respectively. The dotted lines represent the assay cutoffs for each antibody assay. This figure has been modified with permission from He et al.11. Please click here to view a larger version of this figure.
Table 1: Raw CPS counts (left half of the plate). Raw CPS counts acquired from the left half of an assay plate. Each sample is performed in duplicate. Each CPS count within the same column (A1, A2, A3, etc.) represents the reading data from the 10 spots in the same well (corresponding linker numbers are marked). Examples of bad duplicates are highlighted in grey, as seen in row D-linker 3 column 5 and column 6. Please click here to download this Table.
Table 2: Arrangement of the Table 1 data, sorted with six linkers. CPS values from Table 1 were rearranged, calculating the mean values for duplicate readings of CPS and deleting unused values for linkers 4-7. The values of the internal standard high positive, low positive, and negative controls of each autoantibody assay are marked in dark bold. PC, positive control. NC, negative control. Please click here to download this Table.
Table 3: Results of index values. Index values for each sample for all six antibodies were calculated against the corresponding positive and negative controls. An index value greater than the cutoff value was defined as positive, marked in dark bold. The bad duplicate CPS values in Table 1, row D-linker 3-columns 5 and 6, led to a positive IA-2A index value of sample 11 (highlighted in grey), which was probably a false-positive result. Please click here to download this Table.
Table 4: Assay cutoff, sensitivity, and specificity for GADA, IA-2A, ZnT8A, IAA, and TGA among 880 ASK samples, including 325 IAbs positive samples and 555 all antibodies negative samples. The optimal cutoff index value of GADA, IA-2A, ZnT8A, IAA, and TGA assay was set to at least the 99th percentile of the 555 normal control samples. Please click here to download this Table.
Discussion
The intervention for T1D has entered the pre-T1D era, with ongoing national and international large-scale screening programs for T1D and booming clinical trials being applied in stage 1 T1D to abrogate or slow the progression to clinical T1D12,13. Measurements of four IAbs using the current standard RBA with a single IAb assay format is laborious and inefficient for a mass screening program. With the urgent demand for a high-throughput multiplexed IAbs assay, multiplexed ECL assay, 3-Screen ICA ELISA, and antibody detection by agglutination-PCR (ADAP) have emerged as currently competitive technologies. In the Fr1da study of screening for T1D in a population of young children in Germany, 3-Screen ICA ELISA combining 3 IAbs (GADA, IA-2A, and ZnT8A) was used as the main test14. A major limitation of 3-Screen ICA ELISA is the lack of IAA, which is mostly the first IAb to appear with a high prevalence in young children with T1D. Furthermore, the assay is not able to distinguish which of the three IAbs is positive from a positive signal, and every positive sample needs to be repeated with three single assays for confirmation. ADAP15 combines GADA, IA-2A, and IAA and illustrated high assay sensitivity and specificity in the IASP workshop but lacks data regarding how predictive it is in population-based screening for T1D studies, which the IASP workshop is not able to determine. The multiplex ECL assay in the present study is built on the platform of a single ECL assay that has been validated against current standard RBA in multiple clinical trials like TrailNet9,16, DAISY17,18,19, and the ASK4,20,21 study. The present assay has been validated against both RBA and single ECL assay using 880 serum samples from participants of the ASK study. As shown in Figure 2 and Figure 3, antibody levels between 6-Plex and single ECL or between 6-Plex and RBA were mostly congruent with each other for the positivity of antibodies. The coincidence rates of IAbs tested by RBA and 6-Plex were 91.7%-97.8%, and the coincidence rates of IAbs tested by single ECL and 6-Plex were 95.3%-99.2%. Discordance between the ECL assay and RBA was mainly from those with single IAb. The levels of IAbs tested by 6-Plex and single ECL (r = 0.6431-0.9434, all p < 0.0001) and 6-Plex and RBA (r = 0.5775-0.8576, all p < 0.0001) were well correlated as well. TGA tested by 6-Plex assay were highly correlated with the results of both the RBA (99.4%) and single ECL assay (99.4%). Additionally, COVID-19A tested by 6-Plex reached 99.8% compliance with the results of the single ECL assay.
As a result, the 6-Plex ECL assay has formally been accepted as the primary screening method for the ongoing ASK study and replaced the standard RBA22. This assay demonstrated its excellent sensitivity and specificity with higher throughput, lower cost, and smaller volume of serum compared with standard RBA.
It has been documented that, in the T1D screening study, single IAb detected by RBA, which took up a large proportion of IAb positivities, were of low affinity, with low disease risk, and resulted in overall low predictive value19,21,23,24,25,26. Such low risk prediction caused huge extra costs of follow-up visits and resulted in difficulties for T1D preventive studies. The established ECL assay platform has been demonstrated in multiple clinical trials to discriminate high-affinity IAbs from low-affinity IAbs generated by RBA and significantly enhance the predictive values for all four IAbs, especially with single IAb positivity16,17,18,21. The multiplex ECL assay technology was built on the platform of the single ECL assay, with this distinct advantage of the detection of high-affinity Abs. In the present study, the 6-Plex ECL assay illustrated its similarity with the corresponding single ECL assays in sensitivity and specificity.
Some limitations and the technical concerns of the multiplex ECL assay using multiple Plex plates have already been covered in previous publications27,28. With six labeled antigens in one well, there may be some influences between different antigens, and the assay background could increase when adding each antibody assay for multiplexing in the same well. A large amount of assay optimization work is needed to adjust the assay conditions, especially for adjusting the final concentrations of each labeled antigen protein based on the checkerboard assay for each antigen, to maintain the sensitivity and specificity for each antibody assay. As mentioned in previous studies27,28, a small number of samples (<1%) result in false positivity on the multiple Plex plate without a clear underlying reason. All positive results should be repeated and confirmed by a single ECL assay as routine laboratory quality assurance; usually, the false positives will be removed. False-negative results caused by the "prozone effect" observed in the previous 7-Plex ECL assay27,28 were not observed in the present study. In the assay described here, we removed the step of the acid treatment of serum samples from the previous protocol; instead, we preheated the serum samples at 56 °C for 30 min (step 5.1.). On the second day of plate washing, we used a washing buffer containing 0.4 M NaCl (step 8.1.) instead of regular washing buffer to wash the plate more stringently. These modifications made the multiplex ECL assay simpler and lowerered background without losing the assay sensitivity.
In conclusion, this assay has outstanding performance for detecting four IAbs, TGA, and COVID-19A simultaneously. The multiplexing feature, as well as the high throughput, low cost, and small serum volume requirement, make large-scale screening for T1D and concomitant diseases much more feasible in the general population. Patients with T1D or any autoimmune diseases have a much higher risk for other autoimmune diseases. The multiplex ECL assay provides an excellent platform to screen for T1D and multiple autoimmune diseases simultaneously.
Disclosures
The authors have nothing to disclose.
Acknowledgements
The study was supported by JDRF grant 2-SRA-2019-695-S-B, 2-SRA-2020-965-S-B, 1-SRA-2017-564-M_N, NIH grant DK32083, and Diabetes Research Center (DRC) grant P30 DK116073.
Materials
| Name | Company | Catalog Number | Comments |
| 5 mM NaCl | ThermoFisher | 1070832 | |
| 96-well Plate Shaker | VWR | 12620-926 | |
| 96-well round bottom plate | Fisher | 8408220 | |
| Biotin | ThermoFisher | PI21329 | |
| Blocker A | MSD | R93AA | |
| Bottle-Top 500 ml-Filter Units | Fisher | 0974064A | |
| Bovine Serum Albumin | Sigma | A-7906 | |
| GAD65 protein | Diamyd Medical | 10-65702-01 | |
| IA-2 protein (aa 605-979) | Creative BioMart | 283309 | |
| MESO QuickPlex SQ120 | MSD | R31QQ-3 | Electrochemiluminescence analyzer |
| PBS 10x | ThermoFisher | 70011044 | |
| PBS 1x | ThermoFisher | 10010023 | |
| Proinsulin protein | AmideBio | 20160118B3 | |
| Read buffer B | MSD | Y0800019 | |
| SARS-CoV-2 RBD protein | Creative Biomart | Spike-190V | |
| Stop Solution | MSD | Y0090019 | |
| Sulfo-TAG | MSD | R91AO-1 | |
| tTG protein | Diarect AG | 15201 | |
| Tween 20 | Sigma | P-1379 | |
| U-Plex 6-Assay SECTOR plate | MSD | Z00U0142E | 6-plex plate |
| U-PLEX Linker 1 | MSD | L0010022 | |
| U-PLEX Linker 10 | MSD | L0100020 | |
| U-PLEX Linker 2 | MSD | L0020015 | |
| U-PLEX Linker 3 | MSD | L0030018 | |
| U-PLEX Linker 8 | MSD | L0080018 | |
| U-PLEX Linker 9 | MSD | L0090014 | |
| ZeBa Column | ThermoFisher | 89892 | Spin columns |
| ZnT8 protein | Research Laboratory | n/a |
References
- Group, T. S. The Environmental Determinants of Diabetes in the Young (TEDDY) Study. Annals of the New York Academy of Sciences. 1150, 1-13 (2008).
- Vehik, K., et al. Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care. 30 (3), 503-509 (2007).
- Insel, R. A., et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 38 (10), 1964-1974 (2015).
- Stahl, M. G., et al. Mass screening for celiac disease: The autoimmunity screening for kids study. The American Journal of Gastroenterology. 116 (1), 180-187 (2021).
- Al-Hussaini, A., Sulaiman, N., Al-Zahrani, M., Alenizi, A., El Haj, I. High prevalence of celiac disease among Saudi children with type 1 diabetes: A prospective cross-sectional study. BMC Gastroenterology. 12, 180 (2012).
- Agardh, D., et al. Clinical features of celiac disease: A prospective birth cohort. Pediatrics. 135 (4), 627-634 (2015).
- COVID-19: Clinical manifestations and diagnosis in children. UpToDate Available from: https://www.uptodate.com/contents/covid-19-clinical-manifestations-and-diagnosis-in-children/print (2021)
- Barron, E., et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. The Lancet Diabetes & Endocrinology. 8 (10), 813-822 (2020).
- Miao, D., et al. Electrochemiluminescence assays for insulin and glutamic acid decarboxylase autoantibodies improve prediction of type 1 diabetes risk. Diabetes Technology & Therapeutics. 17 (2), 119-127 (2015).
- Jia, X., et al. Prevalence of SARS-CoV-2 antibodies in children and adults with Type 1 diabetes. Diabetes Technology & Therapeutics. 23 (7), 517-521 (2021).
- He, L., et al. High-throughput multiplex electrochemiluminescence assay applicable to general population screening for type 1 diabetes and celiac disease. Diabetes Technology & Therapeutics. , (2022).
- McQueen, R. B., et al. Cost and cost-effectiveness of large-scale screening for type 1 diabetes in Colorado. Diabetes Care. 43 (7), 1496-1503 (2020).
- Insel, R. A., Dunne, J. L., Ziegler, A. G. General population screening for type 1 diabetes: has its time come. Current Opinion in Endocrinology & Diabetes and Obesity. 22 (4), 270-276 (2015).
- Ziegler, A. G., et al. 3 Screen ELISA for high-throughput detection of beta cell autoantibodies in capillary blood. Diabetes Technology & Therapeutics. 18 (11), 687-693 (2016).
- Cortez, F. J., et al. Sensitive detection of multiple islet autoantibodies in type 1 diabetes using small sample volumes by agglutination-PCR. PLoS One. 15 (11), 0242049 (2020).
- Steck, A. K., et al. ECL-IAA and ECL-GADA can identify high-risk single autoantibody-positive relatives in the TrialNet pathway to prevention study. Diabetes Technology & Therapeutics. 18 (7), 410-414 (2016).
- Yu, L., et al. Proinsulin/Insulin autoantibodies measured with electrochemiluminescent assay are the earliest indicator of prediabetic islet autoimmunity. Diabetes Care. 36 (8), 2266-2270 (2013).
- Miao, D., et al. GAD65 autoantibodies detected by electrochemiluminescence assay identify high risk for type 1 diabetes. Diabetes. 62 (12), 4174-4178 (2013).
- Yu, L., et al. Distinguishing persistent insulin autoantibodies with differential risk: Nonradioactive bivalent proinsulin/insulin autoantibody assay. Diabetes. 61 (1), 179-186 (2012).
- Zhao, Z., et al. Higher sensitivity and earlier identification of celiac disease autoimmunity by a nonradioactive assay for transglutaminase autoantibodies. Journal of Immunology Research. 2016, 2904563 (2016).
- Jia, X., et al. High-affinity ZnT8 autoantibodies by electrochemiluminescence assay improve risk prediction for type 1 diabetes. The Journal of Clinical Endocrinology and Metabolism. 106 (12), 3455-3463 (2021).
- He, L., et al. A complete-panel islet autoantibody multiplex ECL assay. Diabetes. 70, 158 (2021).
- Achenbach, P., et al. Mature high-affinity immune responses to (pro)insulin anticipate the autoimmune cascade that leads to type 1 diabetes. Journal of Clinical Investigation. 114 (4), 589-597 (2004).
- Schlosser, M., et al. In insulin-autoantibody-positive children from the general population, antibody affinity identifies those at high and low risk. Diabetologia. 48 (9), 1830-1832 (2005).
- Mayr, A., et al. GAD autoantibody affinity and epitope specificity identify distinct immunization profiles in children at risk for type 1 diabetes. Diabetes. 56 (6), 1527-1533 (2007).
- Siljander, H., et al. Role of insulin autoantibody affinity as a predictive marker for type 1 diabetes in young children with HLA-conferred disease susceptibility. Diabetes/Metabolism Research and Reviews. 25 (7), 615-622 (2009).
- Gu, Y., et al. High-throughput multiplexed autoantibody detection to screen type 1 diabetes and multiple autoimmune diseases simultaneously. Ebiomedicine. 47, 365-372 (2019).
- Jia, X., He, L., Gu, Y., High, H., Yu, L. A high-throughput electrochemiluminescence 7-plex assay simultaneously screening for type 1 diabetes and multiple autoimmune diseases. Journal of Visualized Experiments. (159), e61160 (2020).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved