Method Article
CAM-Delam Assay to Score Metastatic Properties by Quantifying Delamination and Invasion Capacity of Cancer Cells
In This Article
Summary
The CAM-Delam assay to evaluate the metastatic capacity of cancer cells is relatively fast, easy, and cheap. The method can be used for unraveling the molecular mechanisms regulating metastasis formation and for drug screening. An optimized assay for analyzing human tumor samples could be a clinical method for personalized cancer treatment.
Abstract
The major cause of cancer-related deaths is metastasis formation (i.e., when cancer cells spread from the primary tumor to distant organs and form secondary tumors). Delamination, defined as the degradation of the basal lamina and basement membrane, is the initial process that facilitates the transmigration and spread of cancer cells to other tissues and organs. Scoring the delamination capacity of cancer cells would indicate the metastatic potential of these cells.
We have developed a standardized method, the ex ovo CAM-Delam assay, to visualize and quantify the ability of cancer cells to delaminate and invade, thereby being able to assess metastatic aggressiveness. Briefly, the CAM-Delam method includes seeding cancer cells in silicone rings on the chick chorioallantoic membrane (CAM) at embryonic day 10, followed by incubation from hours to a few days. The CAM-Delam assay includes the use of an internal humidified chamber during chick embryo incubation. This novel approach increased embryo survival from 10%-50% to 80%-90%, which resolved previous technical problems with low embryo survival rates in different CAM assays.
Next, the CAM samples with associated cancer cell clusters were isolated, fixed, and frozen. Finally, cryostat-sectioned samples were visualized and analyzed for basement membrane damage and cancer cell invasion using immunohistochemistry. By evaluating various known metastatic and non-metastatic cancer cell lines designed to express green fluorescent protein (GFP), the CAM-Delam quantitative results showed that the delamination capacity patterns reflect metastatic aggressiveness and can be scored into four categories. Future use of this assay, apart from quantifying delamination capacity as an indication of metastatic aggressiveness, is to unravel the molecular mechanisms that control delamination, invasion, the formation of micrometastases, and changes in the tumor microenvironment.
Introduction
Approximately 90% of mortality in cancer patients is caused by the consequences of cancer metastasis, which is the formation of secondary tumors in other parts of the body from where the cancer originally originated1. It is, therefore, of importance to identify metastatic-related mechanisms to find potential targets to suppress the formation of tumor metastases. Subsequently, there is a need for model systems in which the metastatic process can be evaluated.
During metastasis, cancer cells undergo epithelial-to-mesenchymal transition (EMT), a normal cellular process in which epithelial cells lose their adherence and polarity properties and instead acquire an invasive mesenchymal character2. Delamination is part of the EMT process and involves the degradation of laminin in the basement membrane, which is a prerequisite for cancer cells to leave the primary tumor and invade other tissues. The major factors that are upregulated during metastasis formation include matrix metalloproteinases (MMPs), ADAM (a disintergin and metalloproteinase), ADAMTS (ADAM with thrombospondin motifs), and membrane-type MMPs (MT-MMPs)3,4. These factors degrade molecules such as laminin, which is expressed in all basement membranes, to facilitate cell migration and invasion.
The chorioallantoic membrane (CAM) of a fertilized chick egg is a type of basement membrane. Fertilized chick eggs have been used as metastatic models, in which cancer cells have been seeded on the extraembryonic CAM and later metastasis formation observed in the chick embryos5. Moreover, in vivo mouse metastatic models are frequently used, in which cancer cells are implanted in the mice and metastases in various organs are analyzed6. This approach is time-consuming, expensive, and may cause discomfort for the animals. To address this, we have developed the CAM-Delam assay, a faster and cheaper model to evaluate the metastatic aggressiveness of cancer cells. In this model, the ability of cancer cells to degrade the chick CAM (e.g., the delamination capacity) is combined with potential cancer cell invasion into the mesenchyme and used as a measurement of metastatic aggressiveness.
The present article, based on a previous publication7, describes the CAM-Delam assay in detail, from fertilized chick egg handling, cancer cell culture and seeding, dissection, and analyses of CAM samples, to the scoring of the delamination capacity of cancer cells into four categories: intact, altered, damaged, and invasion. We also give examples of how this assay can be used to determine the molecular mechanisms regulating the delamination process.
Protocol
In brief, Figure 1 summarizes the overall steps in the CAM-Delam assay. The below protocol is based on 30 cultured fertilized chicken eggs and the use of two different cancer cell lines seeded separately in three rings/egg and analyzed at four time points.
1. Egg incubation
- Use fertilized chick eggs from a local hatchery, which guarantee over 90% fertilization
NOTE: The eggs should not have been stored for more than 4 days at room temperature (RT). In Sweden, ethical permission for the experimental use of embryonic chickens is only required from embryonic day (E) 14 and beyond. - If needed, carefully mechanically wipe off any dirt or feathers on the eggs using a dry paper towel or wet in water.
- Clean and sterilize the egg incubator with 70% ethanol before use (every time).
- Place the desired number of eggs in egg trays and incubate the chick eggs horizontally in an egg incubator at 37.5-38 °C with 70% humidity. Consider this as incubation day 0 (Figure 1A).
2. Preparation of weighing boat, plastic boxes, and dissection instruments
- Use 70% ethanol to sterilize 30 weighing boats and 30 small plastic boxes (~0.4 L) with transparent caps.
- Dry the weighing boats and boxes in a laminar hood overnight (ON) and store in a closed plastic box until further use on incubation day 3.
- Sterilize 2 L of distilled or deionized H2O per 30 incubated eggs.
- Sterilize two pairs of scissors and three pairs of forceps by spraying 70% ethanol. Air-dry the instruments and then store them in a sterilized box until incubation day 3.
NOTE: These actions (Steps 2.1-2.4) can be done any day prior to incubation day 3. Use gloves to avoid contamination.
3. Opening the eggs and transfer to the internal humidified chamber
NOTE: Use gloves and a face mask to avoid contamination.
- Add approximately 50 mL of sterilized H2O to the sterilized plastic boxes. Keep the water-filled boxes with closed lids, to avoid contamination, at RT until use.
- On incubation day 3, crack the eggshell using the sharp part of scissors and cut a straight opening in the shell.
- Manually break open the eggshell over a weighing boat and collect the egg white, the yolk, and its attached healthy embryo in the weighing boat (Figure 1B). Look for an intact embryo with a beating heart, intact yolk, and developed blood vessels for the experiment (>95% of the incubated eggs). Discard eggs with a malformed embryo, an embryo without a beating heart, a broken egg yolk, or damaged yolk blood vessels.
- Gently transfer the weighing boat into an internal humidified chamber.
NOTE: For Steps 3.2.-3.4., work as fast as possible to avoid contamination, and if possible, use a laminar hood. - Incubate the internal humidified chamber in the egg incubator (Figure 1C).
- Culture the shell-less eggs from day 3 to day 10 in the egg incubator before being used (see Step 3.6.), and when needed, add water to the incubator to maintain 70% humidity.
- Check the internal humidified chambers through the transparent plastic lid for dead embryos or contaminated shell-less eggs every day, and remove them from the incubator.
4. Preparation of silicone rings
- To prepare silicone rings, cut a silicone tube with an inner and outer diameter of 4 mm and 5 mm, respectively, in ~1 mm thickness, preferably using a paper cutter (Figure 1D).
- Transfer the silicone rings to small glass bottles (Figure 1D), cover with metal foil, and sterilize them using an autoclave or similar.
NOTE: Avoid repeated autoclaving of unused silicone rings that might be contaminated, since such treatment decreases the capacity of the silicone rings to attach to the membrane, with subsequent leakage of the cancer cell/collagen/RPMI solution outside of the rings. - Store the sterile silicone rings at RT.
NOTE: This step can be done any day prior to incubation day 10.
5. Preparation of cancer cells
NOTE: Solutions such as cell culture medium, trypsin, and 1x PBS are stored at 4 °C and should be heated to 37 °C in a water bath before adding to the cells. After heating, rinse the bottles in 70% ethanol and dry before use.
- Culture the cancer cell lines of interest in the relevant culture medium in a cell culture incubator at 37 °C in the presence of 5% (v/v) CO2.
NOTE: Here, U251-GFP glioblastoma and PC-3U-GFP prostate cancer cells were cultured in complete RPMI medium-RPMI medium supplemented with 10% (v/v) fetal bovine serum (FBS) and 1% (v/v) penicillin-streptomycin (PS). - Plan the culture of cancer cells so that approximately 50 × 106 cancer cells are ready for harvesting on incubation day 10 of the CAM-Delam assay.
- Change the cell culture medium every 2-3 days, or when the medium color changes from pink to orange/yellow.
- Optional: Induce hypoxia by treating the cancer cells with CoCl2 to investigate the molecular mechanistic effects on delamination 1 day before cell harvesting.
CAUTION: CoCl2 has moderate toxicity. Handle with care in a fume hood, and follow the manufacturer's instructions.- Prepare fresh 20 mM CoCl2 stock solution by dissolving 0.0258 g in 10 mL of sterilized distilled H20 in a 15 mL conical tube wrapped with aluminum foil to protect from light.
- In a 50 mL conical tube, mix 250 µL of the 20 mM CoCl2 stock solution in 25 mL of RPMI medium supplemented with 1% (v/v) PS (but without FBS) per 15 cm diameter cell culture dish. Vortex gently.
- Remove the complete RPMI medium from the cell culture dishes.
- Wash the cells with sterilized 1x PBS 2x.
- Add 25 mL of RPMI medium with 200 µM CoCl2 cell trypsinization (see Step 5.6).
- On egg incubation day 10, prepare 1 mL of a collagen/RPMI mix (ratio 1:3), containing 250 µL of type I collagen (5 mg/mL) and 750 µL of cell culture RPMI medium supplemented with 10% FBS and 1% (v/v) PS. Keep the collagen/RPMI-mix on ice.
- Trypsinize the cancer cells to isolate the cells by first removing the cell culture medium and wash 2x with 1x PBS.
- Add 3 mL of trypsin solution (0.05%) per 15 cm diameter cell culture dish and incubate for 2-3 min in a cell culture incubator until the cells detach. Use a table-top, inverted microscope to see the detached cells. If needed, gently tap the side of the flask to dislodge remaining clustered or attached cells.
- Inactivate trypsin by adding 5 mL of complete RPMI medium to each cell culture dish and collect the cell suspension from all cell culture dishes into a 50 mL tube.
- Count the cancer cells by the Trypan Blue exclusion method to distinguish live from dead cells using a cell counter.Add 10 µL of the cell suspension to 10 µL of 0.4% trypan blue stain. Mix the sample by pipetting up and down a few times, and then load 10 µL of the cell mixture per chamber into the sample slide in the cell counter. Repeat cell counting 3x to verify the cell number/mL.
- Calculate and centrifuge the correct volume cell culture suspension containing 50 x 106 live cancer cells in a 50 mL tube at 500 × g for 5 min.
- Discard the supernatant and mix the cell pellet with 1 mL of the collagen/RPMI mix. As collagen is a gelatinous material, mix the cells very slowly and carefully with a 1 mL pipette to avoid losing cells in the formation of bubbles.
- Keep the prepared cell suspension on ice and bring it to the egg working area.
NOTE: Avoid keeping the prepared cancer cells on ice for too long. The prepared cancer cells must be placed on the CAM within 15-25 min.
6. Seeding the cancer cells on the CAM
NOTE: Use gloves and a face mask to avoid contamination.
- At incubation day 10, take out the internal humidified chambers with the incubated shell-less eggs from the incubator.
NOTE: Approximately 90% of the initial number of the incubated eggs can be used; see Figure 2. - Open the internal humidified chamber and place up to six silicone rings on the CAM using sterilized forceps.
NOTE: Avoid placing the silicone rings near each other or close to bigger blood vessels. - Mix the cancer cell suspension by pipetting to get an even distribution of cancer cells, and add 20 µL (1 × 106 cells) of the prepared cancer cell suspension inside a silicone ring (Figure 1E). When adding the cells, keep the pipette tip above the CAM to make sure not to damage the membrane.
NOTE: According to previous findings7, different cancer cells can be seeded in separate rings on the same CAM. - Close the internal humidified chamber and put it in the egg incubator.
7. Isolation of the CAM with associated cancer cells
- After 14 h, 1.5 days, 2.5 days, and 3.5 days of incubation, take out approximately seven internal humidified chambers and open the lids one at a time.
- With a pair of scissors, dissect the cultured cancer cells attached to the CAM (so-called CAM-Delam samples) by cutting outside the silicone ring (Figure 1F).
- Immediately transfer the isolated CAM-Delam sample using forceps to 4% paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB) in a Petri dish for fixation of the tissue. Keep on ice or at 4 °C for 1 h.
NOTE: Store 4% PFA solution at 4 °C for a maximum of 5 days. CAUTION: PFA is toxic. When handling PFA powder and PFA solutions, use a fume hood and wear a face mask and gloves. Avoid inhaling powder or solution vapors. Follow the manufacturer's instructions. - Decapitate the chicken embryos and discard as biological waste in specific bins.
- Remove the 4% PFA solution and add 30% sucrose in 0.1 M PB to the CAM-Delam samples and equilibrate at 4 °C for 1 h.
- Under a dissection microscope, carefully remove the silicone ring using forceps. With a pair of scissors, cut the CAM-Delam sample in a rectangular shape with the cancer cells in the middle (Figure 1G).
- With a pair of forceps, transfer the CAM-Delam samples to frozen section medium in a Petri dish to remove excess sucrose and then to embedding molds in frozen section medium.
- Under a dissection microscope, position the CAM-Delam sample in a U-shape in a vertical direction in the embedding molds using any needle-like instrument (Figure 1G).
- Freeze and store the CAM-Delam samples at −80 °C.
8. Sectioning CAM-Delam samples
- Section the frozen CAM-Delam samples at 10 µm on 5-6 consecutive slides using cryosectioning.
- Store the slides with sections at −80 °C or use directly for immunohistochemistry staining.
9. Immunohistochemistry staining
- Bring the slides from −80 °C to RT for 5 min before starting the immunoprotocol.
- Make a line with a hydrophobic marker on the slides where the sections end. Let them dry for a few minutes.
- Place the slides in a humidified (H2O) chamber and cover the sections with ~200-500 µL of blocking solution (10% fetal calf serum and 0.1% sodium azide in Tris-buffered saline with 0.1% Triton X-100 [TBST]) and incubate for 15-30 min.
NOTE: From this step onwards, do not let the slides dry at any time. - Pour off the blocking solution and replace it with 100-150 µL of the primary antibody of interest diluted in the blocking solution and incubate ON at 4 °C. Preferably use rabbit anti-laminin-111 antibody to define the basal lamina together with a cancer cell marker, if not using GFP or other fluorescent-tagged cancer cell lines.
NOTE: Here, a rabbit anti-von Willebrand Factor was also used to detect blood vessels in the CAM. - Prepare three glass cuvettes filled with TBST buffer.
- Pour off the primary antibody solution, transfer the slides to the glass cuvettes, and wash at least 3x for 5 min each in TBST.
- Remove excess TBST from the slide and from the hydrophobic barrier area with a soft paper tissue.
- Cover the slide with 100-150 µL of a suitable secondary fluorescent antibody diluted in blocking solution combined with 4',6-diamidino-2-phenylindole, dihydrochloride (DAPI) and incubate in the dark at RT for 1 h.
- Pour off the secondary antibody solution, transfer the slides to the glass cuvettes, and wash at least 3x for 5 min each in TBST.
- Remove excess TBST from the slide and from the hydrophobic barrier area with a soft paper tissue.
- Mount the slides by putting 1-2 drops of fluorescent mounting medium on the slide, and gently place a glass coverslip, avoiding air bubbles.
- Let the slides dry for at least 1 h at 4 °C before analyzing, and store the slides 4 °C.
10. Microscopy imaging and delamination scoring
- Photograph the sections using an epifluorescence microscope equipped with a digital camera, preferably at 10x magnification (Figure 1H).
- Analyze the sections using the following CAM-Delam scoring categories (Figure 3):
- Look for intact basal lamina without visible alterations.
- Look for altered but undamaged basal lamina.
- Look for damaged basal lamina without cell invasion.
- Look for damaged basal lamina with cell invasion.
Results
Figure 1 presents key steps in the CAM-Delam assay7. The use of internal humidified chambers (Figure 1C) significantly improved the survival rate of the chick embryos from <50% up to 90% at incubation day 10 and from ~15% up to 80% at incubation day 13 (Figure 2).
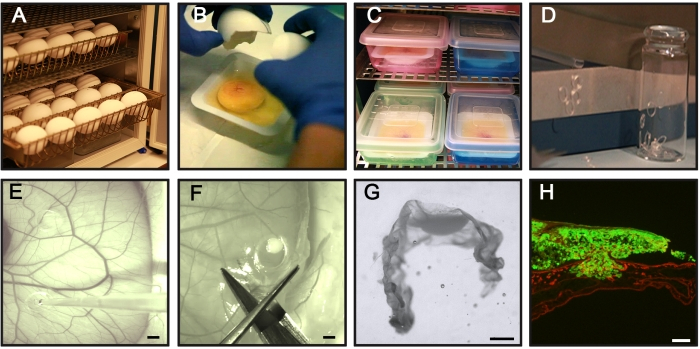
Figure 1: Key steps of the CAM-Delam assay. (A) Incubate fertilized chicken eggs horizontally without rotation. (B,C) On day 3 of incubation, crack the eggs and place them in sterile weighing boats (B), and position the boats in an internal humidified chamber for further incubation (C). (D) Prepare silicone rings. (E) On day 10 of incubation, place the silicone rings on the CAM, and seed 1 x 106 cancer cells inside the rings using a pipette. (F,G) At different time points (hours to days), dissect the CAM with attached cancer cells, (F) fix in PFA, treat with sucrose, position in frozen section medium, (G) and freeze in −80 °C. (H) An example of a sectioned CAM with associated GFP+ cancer cells (green) and laminin immunohistochemistry staining (red). Scale bars = 2 mm (E,F), 1 mm (G), and 100 µm (H). This figure is from Palaniappan et al.7. Abbreviations: CAM = chorioallantoic membrane; PFA = paraformaldehyde; GFP = green fluorescent protein. Please click here to view a larger version of this figure.
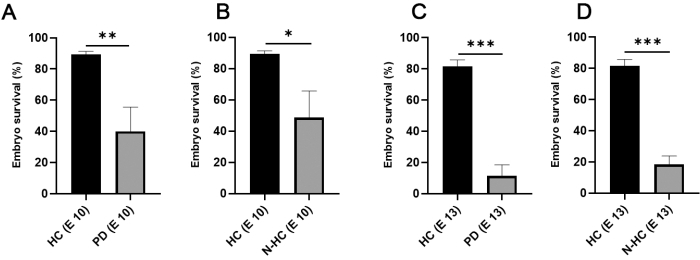
Figure 2: Chicken embryo survival in relation to incubation method. (A,B) On incubation day 10, chicken embryo survival in internal HCs doubled (mean value 89.33; N = 105) compared with incubation in Petri dishes (A; mean value 40; N = 64) and non-humidified chambers (B; mean value 48.67; N = 46). (C,D) On incubation day 13, a high survival rate was still observed using HC (mean value 81.67; N = 105), whereas a major decrease in embryo survival was noticed using PD (C; mean value 11.33; N = 64), and N-HC (D; mean value 18.33; N = 46). Statistical significance was tested using an unpaired two-tailed t-test. The error bars indicate the standard deviation. *p < 0.05, **p < 0.01, ***p < 0.001. This figure was modified from Palaniappan et al.7. Abbreviations: HC = humidified chamber; PD = Petri dishes; N-HC = non-humidified chamber; E X = Incubation day X. Please click here to view a larger version of this figure.
Analyses of different cancer cell lines expressing GFP (U251 glioblastoma, PC-3U prostate, SW620 colon, and A549 lung) using this protocol were reported previously by Palaniappan et al.7. The results from the CAM-Delam assay include differences in morphology of the basal lamina, detected by laminin, and cancer cell invasion, defined as cells that have crossed the chick basal lamina layer into the chick mesodermal layer (Figure 3). These results show that the capacity of cancer cells to degrade basal lamina and invade the mesenchyme can be scored into one of four categories: 1) intact basal lamina without visible alterations (Figure 3B), 2) altered but undamaged basal lamina (Figure 3C), 3) damaged basal lamina without cell invasion (Figure 3D), and 4) damaged basal lamina with cell invasion (Figure 3E). Another observation was that, when cancer cells caused an altered or damaged basal lamina, the CAM was also thickened with an increase of blood vessel formation, defined by antibody staining against von Willebrand's Factor, which is synthesized in blood vessels8 (Figure 4C-H). These two phenotypes, a thickened CAM and increased formation of blood vessels, were not observed when the CAM was intact (Figure 4A,B).
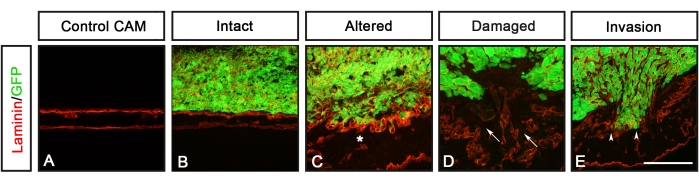
Figure 3: CAM-Delam scoring. (A-E) A CAM-Delam scoring example based on the integrity of the CAM basal lamina, visualized by anti-laminin (red), and potential invasion of GFP-expressing cancer cells (green). (A) Control CAM without cancer cells. (B-E) In the responses to cancer cells, four categories describing the morphology of the basal lamina can be scored: (B) intact laminin, (C) altered but undamaged laminin (indicated by an asterisk), (D) damaged laminin but without cancer cell invasion (arrows), damaged laminin with cell invasion (arrowheads). Scale bar = 100 µm (A-E). This figure is from Palaniappan et al.7. Abbreviations: CAM = chorioallantoic membrane; GFP = green fluorescent protein. Please click here to view a larger version of this figure.
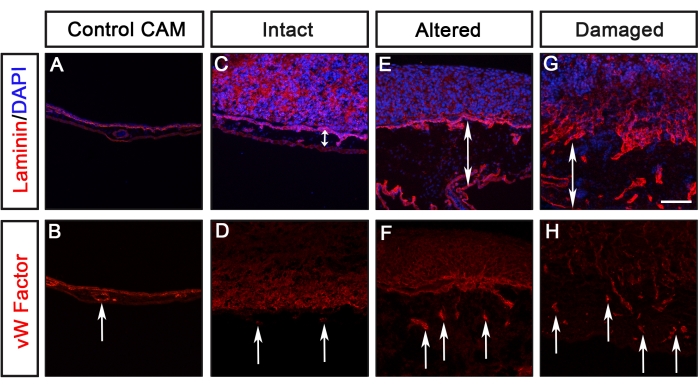
Figure 4: Evaluation of CAM thickening and blood vessel formation. (A-H) An example of visualization of CAM thickening and blood vessel formation, detected by anti-Von Willebrand Factor (red), in response to the lamina of various cancer cell types. (A,B) Control CAM without cancer cells. (C,D) In response to a non-metastatic cancer cell line (scored as Intact), no evident thickening of the mesenchyme or increased blood vessel formation was detected. (E-H) In response to metastatic cancer cells resulting in altered or damaged Laminin, the mesenchyme was thickened (indicated by double arrowheads) and increased blood vessel formation was observed (indicated by arrows). Scale bar = 100 µm. This figure was modified from Palaniappan et al.7. Abbreviations: CAM = chorioallantoic membrane; GFP = green fluorescent protein. Please click here to view a larger version of this figure.
U251 glioblastoma and PC-3U prostate cancer cells are two examples of cancer cell lines with completely different delamination capacities (Figure 5). PC-3U cells induced damaged laminin after 1.5 days, with clear invasion after 2.5 days (Figure 5A). In contrast, U251 cells only induced minor alterations of laminin after 1.5-3.5 days but never caused any visible damage to laminin (Figure 5B).
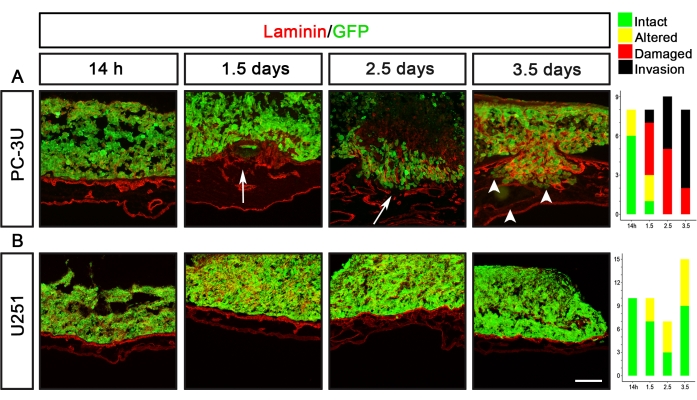
Figure 5: Visualization of the delamination capacity of prostate (PC-3U) and glioblastoma (U251) cancer cells. (A) PC-3U cells induced minor alteration of laminin after 14 h, damage of laminin after 1.5 days (arrow), and the initiation of invasion after 2.5 days, which was increased after 3.5 days (arrowheads). (B) U251 cells caused minor alteration of laminin after 1.5-3.5 days. The right panels show graphs representing the CAM-Delam scoring. The y-axis indicates the number of samples, and the x-axis indicates the time points of culture. Scale bar = 100 µm (A,B). This figure was modified from Palaniappan et al.7. Abbreviations: CAM = chorioallantoic membrane; GFP = green fluorescent protein. Please click here to view a larger version of this figure.
The CAM-Delam assay can be used to define molecular mechanisms that regulate the delamination process. One example is the use of CoCl2 treatment to induce hypoxia with or without the combination of inhibiting matrix metalloproteinases (MMP) using the broad MMP inhibitor GM6001 (Figure 6). After CoCl2 treatment, U251 non-metastatic cancer cells acquired the ability to induce delamination and invasive cells, which was suppressed when CoCl2 treatment was combined with the MMP inhibitor GM6001 (Figure 6). Thus, the CAM-Delam assay can be useful when defining molecules and molecular pathways that affect the delamination process.
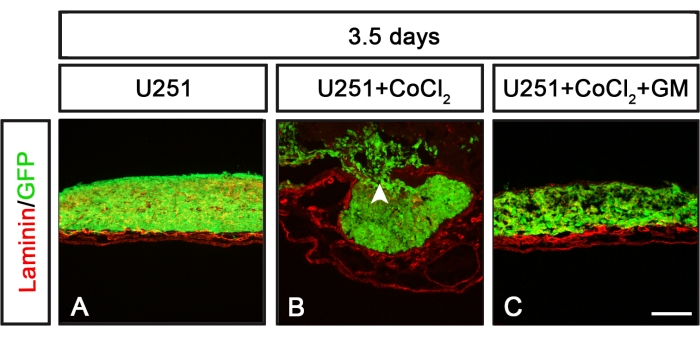
Figure 6: Delamination patterns in response to U251 cells exposed to CoCl2 alone or together with an MMP inhibitor. (A-C) U251 cells cultured for 3.5 days on the CAM during various conditions. (A)U251 cells cultured alone did not induce any damage to laminin. (B) Cultured U251 cells preexposed to CoCl2 (24 h) prior to washing and cell seeding on the CAM induced laminin damage and cell invasion. (C) Pretreatment with a broad-spectrum MMP inhibitor GM6001 (for 1 h), followed by CoCl2 exposure (24 h) before washing and seeding U251 cells on the CAM, suppressed the effect of the CoCl2 treatment, and no obvious laminin damage or cancer cell invasion was detected. Scale bar = 100 µm (A-C). Panels (B) and (C) are from Palaniappan et al.7. Abbreviations: CAM = chorioallantoic membrane; GFP = green fluorescent protein; CoCl2 = cobalt chloride; MMP = matrix metalloproteinase. Please click here to view a larger version of this figure.
Discussion
This paper describes the CAM-Delam assay to evaluate the metastatic aggressiveness of cancer cells, determined by scoring basal lamina modulations and potential cell invasion into the mesenchyme within a period of hours to a few days. A previous technical problem for various CAM assays has been the low survival of chick embryos. This issue was resolved by introducing the use of an internal humidified chamber during embryo incubation, which increased embryo survival from 10%-50% to 80%-90%7. The use of an internal humidified chamber may, therefore, be valuable in CAM-assays in general, as well as in other ex ovo chick experiments.
The presented scoring time points at 14 h, 1.5 days, 2.5 days, and 3.5 days after seeding 1 x 106 cancer cells on the CAM are based on rigorous method development using six different cancer cell lines and are sufficient to distinguish the range from non-delaminating to delaminating-with-invasion capacities of cancer cell lines. A minimum use of four eggs with three rings in each per time point and per cell line is suggested, and this should be repeated at least once or according to experimental designs and statistical requirements. One advantage of the CAM-Delam assay is obtaining informative results regarding the delamination capacity of cancer cells within a few days to estimate the aggressiveness of cancer cells and potential risk for metastasis formation. The rapid delivery of results is facilitated by monitoring the degradation of the basal lamina due to the invading cancer cells and the subsequent microtumors/tumor buds and organ metastases. Traditionally, CAM models have been used to analyze the formation of organ metastases, which takes around 2 weeks to be determined9. By using seven different cancer cell lines, we have previously verified7 that the delamination scoring is linked to the ability of cancer cells to form metastases in rodent models10,11,12,13,14, which supports the predictive value of the CAM-Delam assay. Moreover, mouse models require an even longer time, several weeks up to months, before metastases can be examined15,16. In brief, this developed CAM-Delam assay, focused on scoring delamination capacity and not on examining later tumor formation in the chick embryo, is, therefore, a good complement to existing chicken CAM invasion and mouse tumor models.
A limitation in the CAM-Delam assay may be the unclear visualization of the basal lamina if the cancer cells themselves express laminin. If so, other markers indicating the basal lamina, such as E-cadherin, could be used7. Other CAM invasion studies have used type IV collagen to visualize the CAM and pan-cytokeratin and vimentin to identify invading cancer cells and the formation of microtumors/tumor buds17,18.
Delamination is a normal cellular process, both during development and later in life, which makes it possible for cells to leave an epithelium and migrate to other tissues. Examples of delaminating cells during development are neural crest and olfactory pioneering neurons19,20; later in life, wound healing is dependent on delamination21. During cancer, this process is upregulated in the wrong cells and/or at the wrong time. Thus, the CAM-Delam method can be of use to unravel the molecular mechanisms that regulate delamination, which would be of importance for both basic biological and disease knowledge. Such delamination studies would include adding factors of interest to the cancer cells seeded on the CAM or studying genetically modified cancer cells. One example presented here is CoCl2 pretreatment of the non-metastatic cell line U251 to induce hypoxia, which leads to the induction of a metastatic aggressive capacity that could be suppressed by a broad-spectrum MMP inhibitor. Thus, finding key molecules that control delamination increases the possibility of designing inhibitors to suppress this process. In relation to this, another potential use for the CAM-Delam protocol is in drug screening for the suppression of delamination and cell invasion. Furthermore, in the clinic, the evaluation of cancer severity is a critical component for diagnosis, planning treatment, and care. Currently, the TNM staging system (T, tumor size; N, node-whether the cancer has spread to the lymph nodes; M, distant metastasis) is used to evaluate the severity of the cancer22. The CAM-Delam assay defines an innovative approach to evaluate the aggressiveness of cancer cells and potential risk for metastases formation and might be a useful complement to the TNM staging system. Notable is that TNM staging is based on the analyses of fixed tissue samples, whereas a potential clinical CAM-Delam approach would examine fresh or fresh-frozen tissue in combination with techniques to revive frozen cells23.
Disclosures
The authors have no competing interests to disclose.
Acknowledgements
We thank the following researchers at Umeå University for their help with relevant cancer cell lines and antibodies: L. Carlsson (von Willebrand Factor antibody), J. Gilthorpe (U251), and M. Landström (PC-3U). We also thank Hauke Holthusen in the Gilthorpe lab for the generation of the HEK293-TLR-AAVS1 stable cell line. Work in the Gunhaga laboratory was supported by the Swedish Cancer Foundation (18 0463), Umeå Biotech Incubator, Norrlands Cancerforskningsfond, the Swedish Research Council (2017-01430), and the Medical Faculty at Umeå University.
Materials
| Name | Company | Catalog Number | Comments |
| EQUIPMENT | |||
| Centrifuge | Rotanta 480 R, Hettich zentrifugen | ||
| Countess II FL Automated Cell Counter | Invitrogen | ||
| Cryostat | HM 505 E, Microm | ||
| Digital camera | Nikon DS-Ri1 | ||
| Dissection Microscope | Leica M10 | For CAM-sample dissection and positioning in molds | |
| Egg incubator | Fiem | Many other sources are available. Must be cleaned and sterilized with 70 % ethanol before each use | |
| Epifluorescence microscope | Nikon Eclipse, E800 | Equiped with a digital camera, for scoring delamination and cell invasion. | |
| Fine forceps | Many sources are available | Must be sterilized before use | |
| Freezer -80 °C | Thermo Fisher Scientific | 8600 Series | Model 817CV |
| Inverted microscope | Nikon Eclipse TS100 | For cell culture work | |
| Scissors (small) | Many sources are available | Must be sterilized before use | |
| MATERIALS | |||
| anti-Laminin-111 | Sigma-Aldrich | L9393 | Primary anti-rabbit antibody (1:400) |
| anti-rabbit Cy3 | Jackson Immuno Research | 111-165-003 | Secondary antibody (1:400) |
| anti-von Willebrand Factor | DAKO | P0226 | Primary antibody (1:100) |
Cobalt(II) chloride | Sigma-Aldrich | 232696-5G | CAUTION: moderate toxicity chemical. Handle with care only in fume hood. Follow manufacturers instructions |
| DAPI | Sigma-Aldrich | D9542-10MG | 4',6-diamidino-2-phenylindole, dihydrochloride (1:400) |
| Fertilized chicken eggs | Strömbäcks Ägg, Vännäs, Sweden | Any local egg supplyer | |
| Fetal bovine serum | Life Technologies | 10500-064 | |
| Fluorescence mounting medium | Allent Technologie | S302380-2 | Avoid bubble formation when mounting. Allow to dry at +4 °C |
| Glass chambers for silicon rings | Many sources are available | We used 15 mL glass chambers. Around 20 silicon rings fit in one chamber. Avoid crowding, since the rings may stick together and aggravate work. | |
| Glass coverslips | VWR International | 631-0165 | |
| GM6001 MMP Inhibitor | Sigma-Aldrich | CC1010 | |
| Microscope slides for immunohistochemistry | Fisher Scientific | 10149870 | |
| NEG-50 frozen medium | Cellab | 6506 | |
| Paraformaldehyde | Sigma-Aldrich | 30525-89-4 | CAUTION: highly toxic. Handle with care only in fume hood, follow manufacturers instructions. Use all protective clothing. |
Peel-A-Way Embedding Molds | Polysciences | 18985 | |
| Penicillin–streptomycin | Gibco | 15070063 | |
| Petri dishes | Sarstedt | 83.3903 | 15 cm in diameter for cell culture |
| Plastic box | Esclain | ||
| PureCol EZ Gel Collagen | Cellsystems | 5074-35ML | 5 mg/mL. Gelatinous material. Pipette very slowly and carefully to avoid cells being lost in the bubble formations. |
| RPMI medium | Thermo Fisher Scientific | 21875034 | |
| Silicon rings | VWR International | 228-1580 | Inner/outer diameter: 4/5 mm. Should be sterilized before use. Avoid repeated autoclaving of unused rings. |
| Trypan blue | Fisher Scientific | T10282 | |
| Trypsin | Life Technologi | 15400054 | 0.50% |
| Weighing boats | VWR International | 611-0094 | |
| SOLUTIONS | |||
| Collagen-RPMI media mixture (1 mL) | Compelete RPMI Media 750 µL | ||
| PureCol EZ Gel Collagen 250 µL | |||
| Mix and use immediately | |||
| Complete RPMI media (500 mL) | RPMI Media 445 mL | ||
| FBS 50 mL | |||
| Penicillin–streptomycin 5mL | |||
| Store at 4 °C | |||
| PB (0.2 M; 1 000 mL) | Na2HPO4 (MW 141.76) 21.9 g | ||
| NaH2PO4 (MW 137.99) 6.4 g | |||
| add deionized water up to final volume of 1000ml | |||
| Store in RT | |||
| PFA (4 %) in 0.1 M PB (100 mL) | Deionized water 50 mL | ||
| 0.2 M PB 50 mL | |||
| Paraformaldehyde (PFA) 4g | |||
| heat to 60 °C in water bath | |||
| add 5 M NaOH 25 µL | |||
| Stir to dissolve the PFA powder | |||
| Store at 4 °C | |||
| TBST (1 000 mL) | 50mM Tris pH 7,4 50 mL | ||
| 150mM NaCI 30 mL | |||
| 0,1% Triton X-100 10 mL | |||
| H2O (MQ) 900 mL | |||
| Trypsin (0.05 %; 10 mL) | 1x PBS 9 mL | ||
| 10x Trypsin (0.5 %) 1 mL | |||
| 10 mL in total, Store at 4 °C |
References
- Vasantharajan, S. S., et al. The epigenetic landscape of circulating tumour cells. Biochimica et Biophysica Acta - Reviews on Cancer. 1875 (2), 188514(2021).
- Acloque, H., Adams, M. S., Fishwick, K., Bronner-Fraser, M., Nieto, M. A. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. Journal of Clinical Investigation. 119 (6), 1438-1449 (2009).
- Itoh, Y. Membrane-type matrix metalloproteinases: their functions and regulations. Matrix Biology. 44-46, 207-223 (2015).
- Przemyslaw, L., Boguslaw, H. A., Elzbieta, S., Malgorzata, S. M. ADAM and ADAMTS family proteins and their role in the colorectal cancer etiopathogenesis. BMB Reports. 46 (3), 139-150 (2013).
- Chu, P. Y., Koh, A. P., Antony, J., Huang, R. Y. Applications of the chick chorioallantoic membrane as an alternative model for cancer studies. Cells Tissues Organs. 211 (2), 222-237 (2021).
- MacDonald, I. C., Groom, A. C., Chambers, A. F. Cancer spread and micrometastasis development: quantitative approaches for in vivo models. Bioessays. 24 (10), 885-893 (2002).
- Palaniappan, T. K., Slekiene, L., Jonasson, A. K., Gilthorpe, J., Gunhaga, L. CAM-Delam: an in vivo approach to visualize and quantify the delamination and invasion capacity of human cancer cells. Scientific Reports. 10 (1), 10472(2020).
- Carrillo, M., Kim, S., Rajpurohit, S. K., Kulkarni, V., Jagadeeswaran, P. Zebrafish von Willebrand factor. Blood Cells, Molecules and Diseases. 45 (4), 326-333 (2010).
- Leupold, J. H., Patil, N., Allgayer, H. The chicken egg chorioallantoic membrane (CAM) model as an in vivo method for the investigation of the invasion and metastasis cascade of malignant tumor cells. Methods in Molecular Biology. 2294, 17-26 (2021).
- Jia, Y., et al. Recombinant human endostatin endostar inhibits tumor growth and metastasis in a mouse xenograft model of colon cancer. Pathology and Oncology Research. 18 (2), 315-323 (2012).
- Liu, B., et al. RNAi-mediated inhibition of presenilin 2 inhibits glioma cell growth and invasion and is involved in the regulation of Nrg1/ErbB signaling. Neuro-Oncology. 14 (8), 994-1006 (2012).
- Qin, T., et al. Tumor necrosis factor superfamily 15 promotes lymphatic metastasis via upregulation of vascular endothelial growth factor-C in a mouse model of lung cancer. Cancer Science. 109 (8), 2469-2478 (2018).
- Ren, L., et al. Characterization of the metastatic phenotype of a panel of established osteosarcoma cells. Oncotarget. 6 (30), 29469-29481 (2015).
- Zang, G., Mu, Y., Gao, L., Bergh, A., Landstrom, M. PKCzeta facilitates lymphatic metastatic spread of prostate cancer cells in a mice xenograft model. Oncogene. 38 (22), 4215-4231 (2019).
- Francia, G., Cruz-Munoz, W., Man, S., Xu, P., Kerbel, R. S. Mouse models of advanced spontaneous metastasis for experimental therapeutics. Nature Reviews Cancer. 11 (2), 135-141 (2011).
- Jung, J. Human tumor xenograft models for preclinical assessment of anticancer drug development. Toxicology Research. 30 (1), 1-5 (2014).
- Liu, M., et al. The histone methyltransferase EZH2 mediates tumor progression on the chick chorioallantoic membrane assay, a novel model of head and neck squamous cell carcinoma. Translational Oncology. 6 (3), 273-281 (2013).
- Steinmann, S., et al. DAPK1 loss triggers tumor invasion in colorectal tumor cells. Cell Death & Disease. 10 (12), 895(2019).
- Andrieu, C., et al. MMP14 is required for delamination of chick neural crest cells independently of its catalytic activity. Development. 147 (7), (2020).
- Palaniappan, T. K., Slekiene, L., Gunhaga, L., Patthey, C. Extensive apoptosis during the formation of the terminal nerve ganglion by olfactory placode-derived cells with distinct molecular markers. Differentiation. 110, 8-16 (2019).
- Thiery, J. P., Acloque, H., Huang, R. Y., Nieto, M. A. Epithelial-mesenchymal transitions in development and disease. Cell. 139 (5), 871-890 (2009).
- Pineros, M., et al. Essential TNM: a registry tool to reduce gaps in cancer staging information. The Lancet Oncology. 20 (2), 103-111 (2019).
- Walsh, A. J., Cook, R. S., Sanders, M. E., Arteaga, C. L., Skala, M. C. Drug response in organoids generated from frozen primary tumor tissues. Scientific Reports. 6, 18889(2016).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved