Method Article
Models of Murine Vaginal Colonization by Anaerobically Grown Bacteria
In This Article
Summary
The protocol presents a mouse model of vaginal colonization with anaerobically cultured human vaginal bacteria. We focus on Gardnerella vaginalis, while including suggestions for Prevotella bivia and Fusobacterium nucleatum. This protocol can also be used as a guide for vaginal inoculations and viable recovery of other anaerobically grown bacteria.
Abstract
The mammalian vagina can be colonized by many bacterial taxa. The human vaginal microbiome is often dominated by Lactobacillus species, but one-in-four women experience bacterial vaginosis, in which a low level of lactobacilli is accompanied by an overgrowth of diverse anaerobic bacteria. This condition has been associated with many health complications, including risks to reproductive and sexual health. While there is growing evidence showing the complex nature of microbial interactions in human vaginal health, the individual roles of these different anaerobic bacteria are not fully understood. This is complicated by the lack of adequate models to study anaerobically grown vaginal bacteria. Mouse models allow us to investigate the biology and virulence of these organisms in vivo. Other mouse models of vaginal bacterial inoculation have previously been described. Here, we describe methods for the inoculation of anaerobically grown bacteria and their viable recovery in conventionally raised C57Bl/6 mice. A new, less stressful procedural method for vaginal inoculation and washing is also described. Inoculation and viable recovery of Gardnerella are outlined in detail, and strategies for additional anaerobes such as Prevotella bivia and Fusobacterium nucleatum are discussed.
Introduction
In mammals, the vagina is home to a consortium of bacterial species. The human vaginal microbiome is unique among mammals in its abundance of members of the genus Lactobacillus and corresponding low vaginal pH (3.8-4.5)1,2,3,4. Disruption of this Lactobacillus dominance is associated with a variety of negative health outcomes.
In bacterial vaginosis (BV) there are fewer lactobacilli and an increased abundance of diverse anaerobic bacteria, such as Gardnerella vaginalis and Prevotella bivia5,6. Women with BV are at increased risk of sexually transmitted infections7,8,9, infertility10, pregnancy losses11, preterm birth12,13,14, intrauterine infections15, cervical infections16, and cancer16,17,18. BV is also associated with a higher likelihood of vaginal colonization by potentially pathogenic bacteria, such as Fusobacterium nucleatum1,19,20, a common isolate from amniotic fluid infections21.
Due to the importance of anaerobic bacteria in human vaginal health, there is a need for animal models that can be used to investigate the biology and pathogenesis of these organisms. Here, we describe methods for vaginal inoculation and viable recovery of Gardnerella vaginalis in estrogenized mice and suggest additional strategies for Prevotella bivia and Fusobacterium nucleatum. Other models of murine vaginal colonization have previously been described, but these have focused on the inoculation and recovery of facultative anaerobic bacteria such as Group B Streptococcus22 and Neisseria gonorrhoeae23 that were cultured aerobically. Inoculation and recovery of obligate anaerobes can be successfully accomplished with appropriate experimental strategies. We discuss approaches for the viable recovery of several bacterial taxa and suggest empirical evaluation of conditions for the viability of additional species/strains of interest.
The classical method of estimating colonization by live bacteria is the recovery of colony-forming units (CFUs). In conventionally raised mice with their own endogenous microbiota, this requires recovery on agar media that selects against members of the endogenous vaginal microbiota while still allowing the inoculated strain of bacteria to grow. Here, we use a streptomycin-resistant isolate of G. vaginalis24 that can be selectively recovered on a streptomycin-containing agar medium. To ensure the medium is sufficiently selective and, conversely, that streptomycin-resistant bacteria are not present in the endogenous microbiome, vaginal lavages collected just prior to inoculation should be plated on selective (streptomycin-containing) agar.
The best method for inoculum preparation may vary among species and strains of bacteria. Prior to the start of experiments in mice, preliminary work should be performed to determine preferable conditions for the culture medium, growth endpoint, and preparation of inoculum, as well as the susceptibility to oxygen and viability in PBS. In the case of more oxygen-sensitive bacteria, alternate preparations of the inoculum can be considered (e.g., in an anaerobic culture medium with an appropriate vehicle control group)25,26.
Protocol
All animal experiments were approved and conducted in accordance with institutional guidelines and the Guide for the Care and Use of Laboratory Animals of the University of California San Diego (UCSD, Protocol Number: S20057, 2020-onward) and before that at Washington University, St. Louis (Protocol 20110149, up to 2020).
NOTE: The protocol below utilizes conventionally raised female C57Bl/ mice, age 6-11 weeks at the time of inoculation. Please note vendor information and specific age ranges used previously in the relevant cited work.
1. Preparation and administration of β-estradiol 17-valerate
NOTE: β-estradiol 17-valerate is a carcinogen and a reproductive toxin that can be absorbed through the skin27,28. Wear proper PPE during the handling of powder and liquid solution. It is also an aquatic toxin29; dispose of it properly in an appropriately sealed and labeled container.
- Filter sterilize up to 50 mL of sesame oil through a 0.22 µm filter using a 50 mL conical tube vacuum filter system. Open only in a biosafety cabinet to maintain sterility.
- Calculate the volume of β-estradiol valerate solution needed for the experiment: 200 µL per mouse plus 2-3 mL extra to account for sample loss during syringe filling (e.g., a 10-mouse experiment requires 4 mL in total). Calculate the amount of β-estradiol valerate needed to make a 5 mg/mL solution and weigh it directly in a 14 mL round-bottomed tube.
- Cap the 14 mL tube tightly and vortex to break up clumps in the powder (this will allow the β-estradiol valerate to dissolve more quickly). Add sterile-filtered sesame oil to the 14 mL tube so that the final concentration of β-estradiol valerate is 5 mg/mL.
- Place the tightly capped 14 mL tube on a rotator at 37 °C for 30-40 min, until the solid powder completely dissolves. Visually confirm the homogeneity of the solution before injection into the mice. Incubation at 37 °C decreases the viscosity of the sesame oil, making it easier to inject.
- Draw up β-estradiol valerate solution into a 1 mL syringe (without a needle). To do this without introducing bubbles, first, draw up a little of the solution, then expel gently while keeping the tip of the syringe in the sesame oil. Draw up the solution again slowly, slightly past the 100 µL mark.
- Place a 25 G x 5/8 in needle onto the syringe. Then, prime the needle by slowly expelling the sesame oil solution until it starts to come out of the needle tip. Expel the solution until the syringe is at the 100 µL mark. Prepare one syringe per mouse.
- Administer the β-estradiol 17-valerate by intraperitoneal injection at 48 h or 72 h prior to inoculation. Inject each mouse with 100 µL of the 5 mg/mL β-estradiol valerate solution (0.5 mg β-estradiol valerate/mouse), as previously described22,30. Store the remaining solution at 4 °C for 72 h until the next injection.
- Administer the β-estradiol valerate solution again on the day of inoculation, immediately prior to vaginal inoculation of the mice. Remove the solution from 4 °C and place it on a rotator at 37 °C for 30 min before injection to allow the sesame oil to warm and become less viscous. Proceed with injections as described in step 1.7.
2. Preparation of inoculum
NOTE: This section contains the general steps for the preparation of inocula from anaerobically grown streptomycin-resistant Gardnerella vaginalis JCP8151B-SmR24,25,31,32. All steps in this section should be done in an anaerobic chamber.
- Cycle any reagents, including prepared NYC III broth, agar, and PBS, into an anaerobic chamber to equilibrate at least 24 h prior to use.
NOTE: Here, a vinyl anaerobic chamber equilibrated with 5% hydrogen gas mix was used. The internal oxygen concentration typically rests at 0 ppm, although there can be low-level transient increases (10-25 ppm) when cycling materials into the chamber. - Two days prior to vaginal inoculation, streak out Gardnerella from a frozen glycerol stock onto an NYC III agar plate in the anaerobic chamber. Use a small container (such as an empty pipette tip box) filled with dry ice to keep the glycerol stock frozen during transport in and out of the chamber.
- 1 day prior to vaginal inoculation, use 5-10 individual Gardnerella colonies from the plate to inoculate 10 mL of NYC III broth. Allow the liquid culture to grow overnight for 16 h at 37 °C. Include a second 1 mL aliquot of the culture medium that is left uninoculated and incubate it alongside the inoculated culture to confirm that the medium is uncontaminated.
- Prepare the inoculum from liquid culture as described below. Perform the inoculum preparation in the anaerobic chamber as much as possible.
- Determine the culture optical density (OD) by adding 200 µL of culture to 800 µL of fresh media in a 1.5 mL cuvette (1:5 dilution). Use a spectrophotometer to measure the density (OD) of the diluted culture at a wavelength of 600 nm (use a separate cuvette with fresh media alone as the blank). Multiply the OD reading by 5 to get the actual OD600 of the 16 h culture.
- Calculate the volume of inoculum needed for the experiment. For example, to infect 15 mice with an inoculum of 20 µL per mouse, 15 x 20 µL = 300 µL of inoculum is needed. Prepare approximately 25% more inoculum than needed, so for 15 mice, prepare 300 µL + (0.25 x 300 µL) = 375 µL of inoculum.
- Using the OD600 determined in step 2.4.1., calculate the volume of the 16 h culture that needs to be spun down and resuspended to get an OD600 = 5.0 inoculum in the volume calculated in step 2.4.2. For example, if the OD600 of the culture is 1.5, and the inoculum volume needed is 375 µL, then the volume of culture to be spun down is (375 µL x 5)/1.5 = 1250 µL.
- Pipette the volume of culture calculated in step 2.4.3. into a sterile microcentrifuge tube. Pellet the bacteria by centrifuging at 16,000 x g for 1-3 min.
- Remove the supernatant and resuspend the pellet in anaerobic PBS at the volume calculated in step 2.4.2. The inoculum will now be at a calculated OD600 of 5.0. If applicable, also prepare a tube of PBS to serve as a vehicle control for mice in the uninfected control group.
- Prior to removing the inoculum tube from the anaerobic chamber, prepare a serial dilution series from 1:10 through 1:10 x 106 in PBS. Spot plate 5 replicates of each dilution onto an agar plate using a multichannel pipet to determine the CFU of the inoculum. This is the pre-inoculation CFU.
3. Vaginal pre-lavage and inoculation with Gardnerella
- Prepare vaginal lavage/wash tubes in the anaerobic chamber (this can be done at the same time as inoculum preparation in step 2.4.). Pipette 70 µL of sterile, anaerobic PBS into 1.5 mL microcentrifuge tubes labeled with mouse numbers. Prepare one wash tube per mouse. Tightly close the tubes and cycle them out of the anaerobic chamber.
- Perform a vaginal lavage on each mouse with 50 µL anaerobic PBS from the individual tubes prepared in step 3.1. These vaginal lavages are the pre-inoculation washes and can be used for downstream measurements and assays.
- After administration of the second β-estradiol valerate injection (step 1.8), place each mouse on top of a cage or a clean hard surface that it can grip with its forepaws. Gently grip the middle part of the tail and lift so that the hindquarters are slightly elevated and the vaginal opening is exposed.
- Using a pipette and p-200 filter tip, draw up 50 µL of PBS from the wash tube corresponding to the appropriate mouse. Gently insert the pipette tip into the vaginal lumen ~2-3 mm, and pipette the 50 µL volume in and out gently, 3x-4x.
- Transfer the collected wash material (~50 µL) back into the same wash tube it came from, pipetting up and down in the wash tube that still contains the remaining 20 µL of PBS. If there is excessive mucus and the tip gets clogged, use a clean p-200 tip to collect the remaining material and place it into the same wash tube.
- Vaginally administer 20 µL of the concentrated bacterial suspension or vehicle control into the vagina of each mouse. Inoculate each mouse immediately following the vaginal lavage for that mouse (i.e., perform lavage and inoculation in sequence for each mouse before moving on to the next). To simplify this process, use two p200 pipettes-one set to 50 µL for lavages and the other to 20 µL for inoculations.
- Restrain the mouse as described in step 3.2.1. Using a p-200 tip, pipette 20 µL of bacterial suspension into the vagina. For co-inoculation experiments using two different bacterial species/strains, use 10 µL of each bacterial suspension and avoid mixing the two strains prior to inoculation.
NOTE: The total inoculation volume should not exceed 20 µL to avoid spillover out of the vagina. - Continue to hold up the hind end of each mouse for 10-20 s to prevent the administered volume from immediately pooling at the vaginal introitus. Then, place the mouse in a new cage/receptacle or with cage mates that have already been inoculated.
- Repeat step 3.2. and step 3.3. for each mouse. Never place mice back in a cage with animals that have not yet been inoculated. This prevents inadvertent exposure of mice to streptomycin-resistant bacteria prior to inoculation.
- Restrain the mouse as described in step 3.2.1. Using a p-200 tip, pipette 20 µL of bacterial suspension into the vagina. For co-inoculation experiments using two different bacterial species/strains, use 10 µL of each bacterial suspension and avoid mixing the two strains prior to inoculation.
- After all the mice have been inoculated, cycle the inoculum tube back into the anaerobic chamber. Prepare and plate another serial dilution as described in step 2.5. This is the post-inoculation CFU. Compare the CFUs from the post-inoculation and pre-inoculation plates to determine if the viability of the inoculum decreased while outside of the anaerobic chamber during the inoculation of the animals.
- Plate the vaginal lavages collected in step 3.2. on selective agar (in this case, 1 mg/mL streptomycin). Incubate the plates for 1-2 days in an anaerobic chamber at 37 °C and examine for growth.
NOTE: Growth indicates that endogenous members of the microbiome are resistant to the selection marker and may, therefore, obscure CFU enumeration of the target organism.
4. Collection of vaginal lavages to determine viable Gardnerella colonization
- Collect the vaginal washes at select time points to analyze vaginal colonization. Collect as described in step 3.2.
- Immediately after collection, cycle the vaginal lavages into an anaerobic chamber. Use 10 µL of each lavage to perform serial dilution and plating onto selective agar as described in step 2.5. Use the CFU counts as a measure of vaginal colonization by Gardnerella (or another anaerobe of interest).
5. Sacrifice, dissection, and tissue homogenization
- Prepare a 5 mL tube for each organ being collected from each mouse (for example, if the vagina, cervix, and uterus are all being collected, prepare three tubes per mouse). Fill each tube with sterile anaerobic 1x PBS (or other homogenization media) in the anaerobic chamber. Use 1 mL of PBS for tubes used to collect vaginas and cervices and 750 µL for tubes used to collect uterine horns.
- Once PBS is added, weigh each individual tube (this is the pre-collection weight and will be used to determine the total weight of each harvested tissue). If a scale is not available in the anaerobic chamber, tightly cap the tubes and cycle them out of the chamber to be weighed, then cycle them back in.
NOTE: Tube preparation and weight collection can be done 1 day in advance of sacrifice, but tubes should be kept in the anaerobic chamber with caps tightly sealed to prevent evaporation. - Prepare a set of vaginal lavage tubes as described in step 3.1. to collect the final lavage at sacrifice. Also, prepare a 50 mL beaker of deionized (DI) water and a second 50 mL beaker of 70%-90% ethanol to use for rinsing and sterilizing standard steel dissection instruments during the sacrifice.
- Cycle out the PBS-filled organ collection tubes from the anaerobic chamber. Keep the tubes tightly closed until the tissues are ready to be added.
- Sacrifice each mouse using IACUC-approved methods. Perform the final lavage as described in steps 3.2.2. and 3.2.3. either immediately prior to sacrifice or immediately thereafter.
- Begin dissection and tissue collection. Spray down the abdomen and back of each mouse with 70% ethanol. Sterilize the scissors in a beaker of ethanol, allow them to dry, and then make a single vertical cut up the mouse abdominopelvic cavity.
- Use sterile forceps to pull back the skin and gently push the intestines out of the body cavity and to the side of the open field, which will expose the reproductive tract.
NOTE: Be careful not to puncture or pierce the intestines as they may harbor streptomycin-resistant bacteria that can confound the experimental results. - To collect the maximum vaginal tissue, break the pubic bone during the necropsy. Using sterile scissors, clip the center of the pubic bone (the pubic symphysis) while being careful not to cut into the vagina. Using two sets of sterile forceps, grab each side of the pelvis and pull the pelvis open laterally, exposing the lower portion of the vagina underneath.
- Use forceps to gently grip the cervix (a harder structure compared to the surrounding tissue). Gently pull up on the cervix to expose the colon, hidden behind and closely connected to the vagina.
- Use small scissors to release the vagina from the external connective tissues. Carefully separate the vagina from the colon and avoid puncturing the intestinal tract. When fully released, flank the vagina at the introitus with scissors and snip to release from the mouse body.
- Maintain a gentle grip on the cervix while pulling up slightly to make the uterine horns more visible. With the other hand, cut directly below the ovaries on the right and left sides to free the uterine horns. Remove the now-disconnected reproductive tract from the body cavity and place it into a sterile Petri plate.
- Using a razor, separate the uterine horns, cervix, and vagina. Place each tissue in its respective labeled collection tube. If cytokines are going to be collected, place the tubes on ice after the addition of tissue.
- Weigh each tube again after the tissue has been added. This is the post-collection weight. Subtract the pre-collection weight from the post-collection weight to get the total weight of the tissue.
- Use a handheld homogenizer to homogenize the organs in their collection tubes. For Gardnerella JCP8151B, perform this step in the biosafety cabinet (aerobically) or the anaerobic chamber.
- Prepare several sets of tubes for washing and aseptic treatment of the handheld homogenizer between samples. Ensure that each set has three 50 mL conical tubes: one filled with DI water, one with 70% ethanol, and the third with 1x PBS. Prepare a separate set of wash tubes for each mouse treatment group.
- To clean the homogenizer, lower the blades into the liquid in each wash tube and turn the instrument to max speed. Hold the homogenizer in each of the three tubes for about 3 s. The order of washing is DI water (to remove physical debris), then ethanol (to sanitize), and finally 1x PBS (to neutralize). Shake the probe onto a paper towel or disposable bench pad to remove excess fluid between each rinse.
- Following initial rinsing, homogenize the tissues by lowering the homogenizer probe to the bottom of the collection tube, trapping the tissue underneath. Turn on the homogenizer to grind the tissue, gently moving the probe up and down about 5x while remaining submerged. Homogenize each organ for about 15-30 s.
- After turning off and removing the homogenizer from the tube, visually inspect the contents to ensure complete tissue disruption. This is especially important for the cervix and vagina, which sometimes fail to be caught by the probe.
NOTE: It is okay if some excess fat on the uterine horns does not fully homogenize. - Repeat steps 5.14.1.-5.14.4., washing and sterilizing the probe between each sample. Switch to a new set of wash tubes for each experimental group.
- Move the tubes with homogenized samples into the anaerobic chamber. To determine the bacterial burden of each tissue, perform brief gentle vortexing of the sample to mix, then remove 100 µL of tissue homogenate for serial dilution and plating on selective agar for CFU determination (the first dilution is 1 x 100). If a lower limit of detection is required, perform spread-plating of 200-250 µL of undiluted homogenate.
Results
A schematic representation of an example experiment shows some of the ways one might carry out the described protocol (Figure 1).
Some animals will carry endogenous microbes in their vaginal microbiomes that will grow on nonselective media. The methods described here rely on streptomycin-resistant isolates of the studied bacterial strains, together with selective media containing streptomycin, to select against endogenous bacteria (Figure 2A,B). It is possible for streptomycin resistance to develop spontaneously, so checking mice prior to infection (day 0 washes) can help establish if this may be a problem.
Recovery of viable bacteria from vaginal washes is accomplished by serial dilution and plating on agar media. A standard petri dish can hold up to a 6 x 6 array of 5 µL spots, allowing enumeration of bacterial titers from six replica spots across six orders of magnitude for each sample if using 10-fold serial dilutions (Figure 3A). This method can be used to enumerate titers of bacteria ranging from Gardnerella to Prevotella and Fusobacterium (Figure 3B-D).
Together, these methods allow one to test hypotheses and make interpretations about bacterial colonization/infection of the female reproductive tract. For example, a comparison of Gardnerella titers in vaginal washes and vaginal tissue homogenates demonstrated concordance between these methods (Figure 4A). Likewise, in a vaginal co-inoculation model, titers of Prevotella in uterine tissue were significantly higher (approximately 20-fold) during Gardnerella co-infection compared to Prevotella mono-infection (Figure 4B). Finally, wild-type versus mutant bacterial strains can be compared in these models to establish the roles played by specific genes and their products in the processes of reproductive tract colonization/infection. For example, a mutant strain of Fusobacterium lacking the ability to transport and consume the carbohydrate sialic acid led to lower levels of colonization by 3 days post-inoculation (Figure 4C).
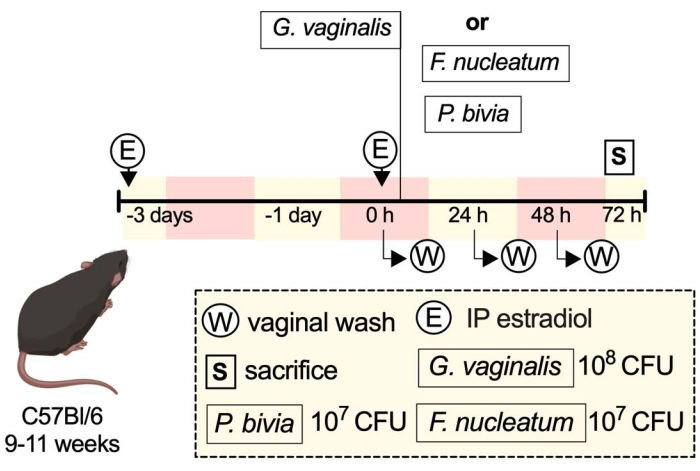
Figure 1: Schematic of an example experiment33. Please click here to view a larger version of this figure.
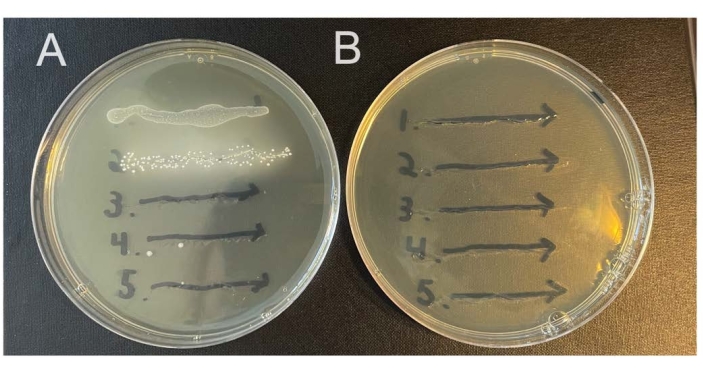
Figure 2: Growth of endogenous murine vaginal microbes on selective versus nonselective agar. Vaginal lavages from five female C57Bl/6 mice (1-5) were streaked onto two different NYC III agar plates and incubated anaerobically. (A) The plate on the left does not contain any antibiotics and permits the growth of endogenous anaerobic bacteria from the murine vaginal tract. (B) The plate on the right contains 1 mg/mL streptomycin and selects against the growth of endogenous bacteria. Please click here to view a larger version of this figure.
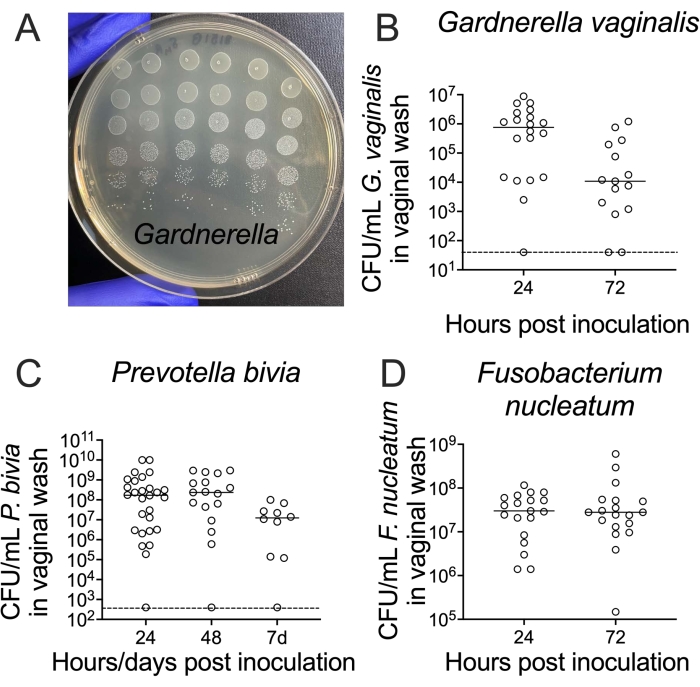
Figure 3: Recovery of viable G. vaginalis, P. bivia, and F. nucleatum CFUs from vaginal washes. (A) CFUs of G. vaginalis JCP8151B-SmR inoculum, replica-plated as a dilution series on NYC III agar. (B-D) Recovery of viable (B) G. vaginalis24, (C) P. bivia25, and (D) F. nucleatum26 from the vaginal lavages of mono-infected animals. For each graph (B-D), the data is combined from two independent experiments, with 10-15 mice per experiment24,25,26. Please click here to view a larger version of this figure.
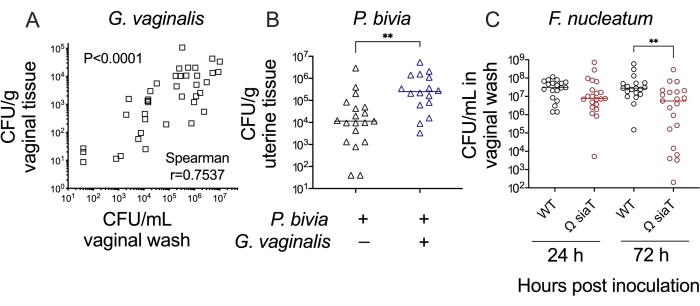
Figure 4: Further analysis of anaerobic bacterial colonization in a murine vaginal inoculation model. (A) G. vaginalis titers in vaginal washes correlate with G. vaginalis titers recovered from vaginal tissue homogenates at 24 h and 72 h post-inoculation (combination of two independent experiments, n = 10 each. Significance determined by Spearman's rank correlation test)24. (B) Co-infection with G. vaginalis leads to increased P. bivia titers in uterine horn tissue when compared to P. bivia mono-infected animals at 48 h post-inoculation (combination of two independent experiments, each with 6-10 mice per group. Significance determined by Mann-Whitney U-test, **p = 0.0017)25. (C) F. nucleatum mutant with disrupted sialic acid transporter (Ω SiaT) has decreased titers in vaginal lavages of mono-infected mice as compared to F. nucleatum wild-type at 72 h post-inoculation (combination of two independent experiments, each with 10 mice per group. Significance determined by Mann-Whitney U-test, **p < 0.01)26. Please click here to view a larger version of this figure.
Supplementary File 1: Examples of methods used for different vaginal bacteria grown under anaerobic conditions. Please click here to download this File.
Discussion
The most significant way the model described here differs from previously published vaginal inoculation models is the use of anaerobically cultured bacteria, which require special considerations for the preparation of viable inoculum and the recovery of bacteria from the mice. Specific steps in the protocol above may vary slightly depending on the species/strain of bacteria used, as suggested in Supplementary File 1. Additionally, this manuscript describes a new procedural method for the administration of bacteria and vaginal washes that requires less experience on the part of the investigator and less restraint for the mice. If the experimenter is not comfortable with the form of restraint described in step 3.2. and step 3.3., the mice can instead be scruffed as previously described34 with or without anesthesia according to the experimental design and institutional IACUC guidelines.
An important caveat to the use of anaerobically cultured bacteria in murine models is that certain steps (such as any involving live animals) must be done outside of the anaerobic chamber. Therefore, the most critical steps in this protocol are those in which the bacteria of interest will be exposed to an aerobic environment, such as during inoculation of the mice. The use of any new anaerobe in this model will require preliminary investigation into its ability to maintain viability upon exposure to oxygen (such as the comparison of pre- and post-inoculation CFUs as described in step 2.5. and step 3.4.). Depending on the degree of oxygen and PBS sensitivity of the organism, different methods of inoculum preparation should be considered (Supplementary File 1, step 2.4.5.). For inoculations using G. vaginalis JCP8151B, we have found that a single tube of inoculum can be prepared in PBS and used to infect all of the mice. If a different anaerobic bacterium is being used that is more sensitive to oxygen, the inoculum can instead be prepared in separate tubes for each mouse so that repeated opening/closing of the tube is not required. Likewise, if the bacterial species of interest is more fastidious/less capable of surviving in PBS than G. vaginalis, inoculation can instead be prepared in culture media. If the medium used contains reducing agents, such as the CDC anaerobe medium used to culture Prevotella bivia (Supplementary File 1, step 2.1. and step 2.2.), then the bacteria will also have increased protection from oxygen exposure.
Alternative methods for homogenization can also be considered, such as bead beating22,35, which is done with the tube cap closed and may, therefore, introduce less oxygen than the handheld homogenizer. Additionally, more oxygen-sensitive organisms may require a longer equilibrium time for media. An oxygen indicator such as resazurin can be used to check that anaerobic conditions have been reached (step 2.1.). Alternative methods such as anaerobic jars may affect results and should be vetted for bacterial viability prior to use.
The oxygen sensitivity and fastidiousness of the experimental organism may also play a role in the successful recovery of viable bacteria from the mouse during lavages/homogenization (Supplementary File 1, step 3.2. and step 5.1.). For highly sensitive organisms, the time between the lavage being taken and plated may lead to a loss of viability and cause an artificially low estimate of bacterial vaginal colonization. To mitigate this effect, collected lavages can immediately be diluted into fresh anaerobic culture media (with reducing agent). Similarly, organ homogenization can be done in fresh culture media rather than PBS to increase bacterial viability and can also be done in the anaerobic chamber itself to minimize oxygen exposure.
Depending on the purpose of a given experiment, the experimenter must pay attention to control groups. To test hypotheses, baseline measures of uninfected control mice that are mock-treated with vehicle alone will help establish if there is induction of chemokines/cytokines or other specific outcomes of infection. The control vehicle used must be the same as the vehicle used for inoculation, so if the bacteria are inoculated in culture media, then uninoculated culture media must be used as the control (Supplementary File 1, step 2.4.5.).
The methods described in this protocol could also be applied to facultative bacteria capable of growing anaerobically but that are traditionally studied using aerobic culture conditions (such as Escherichia coli or Group B Streptococcus). This could be especially relevant for infections by these organisms in body sites that are believed to contain low levels of oxygen, such as the cervix. It may be that a facultative organism more successfully infects sites with less oxygen when the inoculum is prepared anaerobically as compared to aerobically. In such an experiment, the recovery of viable bacteria for CFU enumeration may not require anaerobic conditions.
Disclosures
The authors declare no conflict of interest.
Acknowledgements
We acknowledge Lynne Foster for technical assistance during the development of these models. We appreciate startup funds from the Department of Molecular Microbiology and the Center for Women's Infectious Disease Research (to AL), and the March of Dimes (Basil O'Connor award to AL) which helped support early-stage experiments. The National Institute of Allergy and Infectious Diseases (R01 AI114635) also supported the development of these models.
Materials
| Name | Company | Catalog Number | Comments |
| β-estradiol 17-valerate | Sigma | E1631 | estrogenization of mice |
| 0.22 µm, 50 mL Steriflip vacuum filter | Fisher | SCGP00525 | sterile filtering sesame oil |
| 14 mL tube | Falcon | 352059 | estrogenization of mice |
| 190-proof ethanol | Koptec | V1105M | dissection, tissue homogenization, CDC and Columbia media |
| 1x PBS | Fisher | MT21040CM | vaginal lavage, G. vaginlalis inoculum prep, tissue collection and homogenization |
| 25 G × 5/8 -in needle | BD | 305122 | estrogenization of mice |
| 5 mL tube | Falcon | 352063 | Tissue collection and homogenization |
| Anaerobic chamber | Coy | 7200000 | Growth of anaerobic bacteria |
| Bacto agar | Fisher | DF0140074 | G. vaginalis, F. nucleatum, and P. bivia agar plates |
| Bacto peptone | Fisher | DF0118170 | CDC anaerobic media for P. bivia growth |
| Columbia broth | Fisher | DF0944170 | Columbia media for F. nucleatum growth |
| Defibrinated sheep blood | Hemostat | DSB500 | CDC anaerobic media for P. bivia growth |
| Forceps | Fine Science Tools | 11008-13 | mouse/tissue dissection |
| Glucose | Sigma | G7528 | NYCIII media for G. vaginalis growth |
| Handheld homogenizer | ISC Bio | 3305500 | Tissue homogenization |
| Hemin | Sigma | 51280 | CDC anaerobic media for P. bivia growth, Columbia media for F. nucleatum growth |
| HEPES | Cellgro | 25-060-Cl | NYCIII media for G. vaginalis growth |
| Horse serum | Hemostat | SHS500 | NYCIII media for G. vaginalis growth |
| Hydrogen gas mix (5% hydrogen, 10% CO2, 85% nitrogen) | Matheson | Gas for anaerobic chamber | |
| Isofluorane | VetOne | 502017 | mouse anaethesia at sacrifice |
| L-cysteine | Sigma | C1276 | CDC anaerobic media for P. bivia growth |
| L-glutamate | Sigma | G1626 | CDC anaerobic media for P. bivia growth |
| Milli-Q Water Purifier | Millipore | IQ-7000 | for making media and homogenization |
| NaCl | Sigma | S3014 | NYCIII media for G. vaginalis growth |
| NaOH tablets | Sigma | S5881 | CDC anaerobic media for P. bivia growth |
| Nitrogen gas | Airgas | Gas for anaerobic chamber | |
| p-200 filter tips | ISC Bio | P-1237-200 | vaginal lavages and bacterial inoculations |
| pancreatic digest of casein | Sigma | 70169 | CDC anaerobic media for P. bivia growth |
| Proteose peptone #3 | Fisher | DF-122-17-4 | NYCIII media for G. vaginalis growth |
| Scissors | Fine Science Tools | 14084-08 | mouse/tissue dissection |
| Sesame oil | Fisher | ICN15662191 | estrogenization of mice |
| Single edge surgical carbon steel razor blades | VWR | 55411-050 | tissue dissection |
| Sodium chloride | Sigma | S3014 | CDC anaerobic media for P. bivia growth |
| Spectrophotometer | BioChrom | 80-3000-45 | measuring bacterial OD600 |
| Streptomycin | Gibco | 11860038 | add to agar media to make selective plates |
| Tuberculin slip tip 1ml syringe | BD | 309659 | estrogenization of mice |
| Vitaim K3 | Sigma | M5625 | Columbia media for F. nucleatum growth |
| Vitamin K1 | Sigma | 95271 | CDC anaerobic media for P. bivia growth |
| Yeast extract | Fisher | DF0127-17-9 | NYCIII media for G. vaginalis growth |
References
- Hillier, S. L., Krohn, M. A., Rabe, L. K., Klebanoff, S. J., Eschenbach, D. A. The normal vaginal flora, H2O2-producing lactobacilli, and bacterial vaginosis in pregnant women. Clinical Infectious Diseases. 16, Suppl 4 273-281 (1993).
- Ravel, J., et al. Vaginal microbiome of reproductive-age women. Proceedings of the National Academy of Sciences of the United States of America. 108, Supplement_1 4680-4687 (2011).
- Hill, G. B., Eschenbach, D. A., Holmes, K. K. Bacteriology of the vagina. Scandinavian Journal of Urology and Nephrology. 86, 23-39 (1984).
- van de Wijgert, J. The vaginal microbiome and sexually transmitted infections are interlinked: Consequences for treatment and prevention. PLoS Medicine. 14 (12), 1002478(2017).
- Srinivasan, S., et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One. 7 (6), 37818(2012).
- Shipitsyna, E., et al. Composition of the vaginal microbiota in women of reproductive age-sensitive and specific molecular diagnosis of bacterial vaginosis is possible. PLoS One. 8 (4), 60670(2013).
- Brotman, R. M., et al. Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. The Journal of Infectious Diseases. 202 (12), 1907-1915 (2010).
- Peipert, J. F., et al. Bacterial vaginosis, race, and sexually transmitted infections: does race modify the association. Sexually Transmitted Diseases. 35 (4), 363-367 (2008).
- Wiesenfeld, H. C., Hillier, S. L., Krohn, M. A., Landers, D. V., Sweet, R. L. Bacterial vaginosis is a strong predictor of Neisseria gonorrhoeae and Chlamydia trachomatis infection. Clinical Infectious Diseases. 36 (5), 663-668 (2003).
- Spandorfer, S. D., Neuer, A., Giraldo, P. C., Rosenwaks, Z., Witkin, S. S. Relationship of abnormal vaginal flora, proinflammatory cytokines and idiopathic infertility in women undergoing IVF. The Journal of Reproductive Medicine. 46 (9), 806-810 (2001).
- Ralph, S. G., Rutherford, A. J., Wilson, J. D. Influence of bacterial vaginosis on conception and miscarriage in the first trimester: cohort study. BMJ. 319 (7204), 220-223 (1999).
- Holst, E., Goffeng, A. R., Andersch, B. Bacterial vaginosis and vaginal microorganisms in idiopathic premature labor and association with pregnancy outcome. Journal of Clinical Microbiology. 32 (1), 176-186 (1994).
- DiGiulio, D. B., et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One. 3 (8), 3056(2008).
- Svare, J. A., Schmidt, H., Hansen, B. B., Lose, G. Bacterial vaginosis in a cohort of Danish pregnant women: Prevalence and relationship with preterm delivery, low birthweight and perinatal infections. British Journal of Obstetrics and Gynaecology. 113 (12), 1419-1425 (2006).
- Ádám, A., et al. Culture- and PCR-based detection of BV associated microbiological profile of the removed IUDs and correlation with the time period of IUD in place and the presence of the symptoms of genital tract infection. Annals of Clinical Microbiology and Antimicrobials. 17 (1), 40(2018).
- Di Paola, M., et al. Characterization of cervico-vaginal microbiota in women developing persistent high-risk Human Papillomavirus infection. Scientific Reports. 7 (1), 10200(2017).
- Bullman, S., et al. Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science. 358 (6369), 1443-1448 (2017).
- Brennan, C. A., Garrett, W. S. Gut microbiota, inflammation, and colorectal cancer. Annual Review of Microbiology. 70, 395-411 (2016).
- Hill, G. B. Preterm birth: associations with genital and possibly oral microflora. Annals of Periodontology. 3 (1), 222-232 (1998).
- Hitti, J., et al. Vaginal indicators of amniotic fluid infection in preterm labor. Obstetrics & Gynecology. 97 (2), 211-219 (2001).
- DiGiulio, D. B. Diversity of microbes in amniotic fluid. Seminars in Fetal and Neonatal. 17 (1), 2-11 (2012).
- Patras, K. A., Doran, K. S. A murine model of Group B Streptococcus vaginal colonization. Journal of Visualized Experiments: JoVE. (117), e54708(2016).
- Jerse, A. E., et al. Estradiol-treated female mice as surrogate hosts for Neisseria gonorrhoeae genital tract infections. Frontiers in Microbiology. 2, 107(2011).
- Gilbert, N. M., Lewis, W. G., Lewis, A. L. Clinical features of bacterial vaginosis in a murine model of vaginal infection with Gardnerella vaginalis. PLoS One. 8 (3), 59539(2013).
- Gilbert, N. M., et al. Gardnerella vaginalis and Prevotella bivia trigger distinct and overlapping phenotypes in a mouse model of bacterial vaginosis. The Journal of Infectious Diseases. 220 (7), 1099-1108 (2019).
- Agarwal, K., et al. Glycan cross-feeding supports mutualism between Fusobacterium and the vaginal microbiota. PLOS Biology. 18 (8), 3000788(2020).
- Liehr, J. G. Is estradiol a genotoxic mutagenic carcinogen. Endocrine Reviews. 21 (1), 40-54 (2000).
- Yager, J. D., Davidson, N. E. Estrogen carcinogenesis in breast cancer. The New England Journal of Medicine. 354 (3), 270-282 (2006).
- Adeel, M., Song, X., Wang, Y., Francis, D., Yang, Y. Environmental impact of estrogens on human, animal and plant life: A critical review. Environment International. 99, 107-119 (2017).
- Miner, N. A., Koehler, J., Greenaway, L. Intraperitoneal injection of mice. Journal of Applied Microbiology. 17 (2), 250-251 (1969).
- Lewis, W. G., Robinson, L. S., Gilbert, N. M., Perry, J. C., Lewis, A. L. Degradation, foraging, and depletion of mucus sialoglycans by the vagina-adapted Actinobacterium Gardnerella vaginalis. Journal of Biological Chemistry. 288 (17), 12067-12079 (2013).
- Robinson, L. S., et al. Genome sequences of 15 Gardnerella vaginalis strains isolated from the vaginas of women with and without bacterial vaginosis. Genome Announcements. 4 (5), 00879(2016).
- Adapted from "mouse posterior turn" by BioRender.com. BioRender.com. , Available from: https://app.biorender.com/biorender-templates (2022).
- Machholz, E., Mulder, G., Ruiz, C., Corning, B. F., Pritchett-Corning, K. R. Manual restraint and common compound administration routes in mice and rats. Journal of Visualized Experiments: JoVE. (67), e2771(2012).
- Verollet, R. A major step towards efficient sample preparation with bead-beating. Biotechniques. 44 (6), 832-833 (2008).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved