A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Line Shape Analysis of Dynamic NMR Spectra for Characterizing Coordination Sphere Rearrangements at a Chiral Rhenium Polyhydride Complex
In This Article
Summary
Line shape analysis of NMR spectra collected over a range of temperatures serves as a guide for the rearrangement of inner coordination-sphere atoms at a chiral, eight-coordinate, rhenium(V) polyhydride complex, ReH5(PPh3)2(sec-butyl amine). Line shape analysis is also used to determine the activation parameters ΔH‡, ΔS‡, and ΔG‡ for those atom rearrangements.
Abstract
Dynamic solution nuclear magnetic resonance (NMR) spectroscopy is the typical method of characterizing the dynamic rearrangements of atoms within the coordination sphere for transition metal polyhydride complexes. Line shape fitting of the dynamic NMR spectra can lead to estimates for the activation parameters of the dynamic rearrangement processes. A combination of dynamic 31P-{1H} NMR spectroscopy of metal-bound phosphorus atoms with dynamic 1H-{31P} NMR spectroscopy of hydride ligands may identify hydride ligand rearrangements that occur in conjunction with a phosphorus atom rearrangement. For molecules that exhibit such a coupled pair of rearrangements, dynamic NMR spectroscopy can be used to test theoretical models for the ligand rearrangements. Dynamic 1H-{31P} NMR spectroscopy and line shape fitting can also identify the presence of an exchange process that moves a specific hydride ligand beyond the metal's inner coordination sphere through a proton exchange with a solvent molecule such as adventitious water. The preparation of a new compound, ReH5(PPh3)2(sec-butyl amine), that exemplifies multiple dynamic rearrangement processes is presented along with line shape fitting of dynamic NMR spectra of the complex. Line shape fitting results can be analyzed by the Eyring equation to estimate the activation parameters for the identified dynamic processes.
Introduction
NMR spectroscopy is commonly used to characterize dynamic processes that occur within or between molecules. For many simple intramolecular rearrangements, estimation of ΔG‡ is as straight-forward as measuring the frequency difference, Δν, between two resonances at the slow exchange limit and determining the coalescence temperature for those same resonances (Figure 1)1. The relationship,
ΔG‡ = 4.575 x 10-3 kcal/mol x Tc [9.972 + log (Tc/Δν)]
where Tc is the coalescence temperature for a pair of resonances that represent the slow exchange form of a dynamic sample, can be used to solve for the free energy of activation for such a dynamic rearrangement. More complex dynamic systems require line shape fitting of dynamic NMR spectra or another NMR technique such as two-dimensional exchange spectroscopy (2D-EXSY) or two-dimensional rotating-frame Overhauser effect spectroscopy (2D-ROESY) to estimate activation parameters.
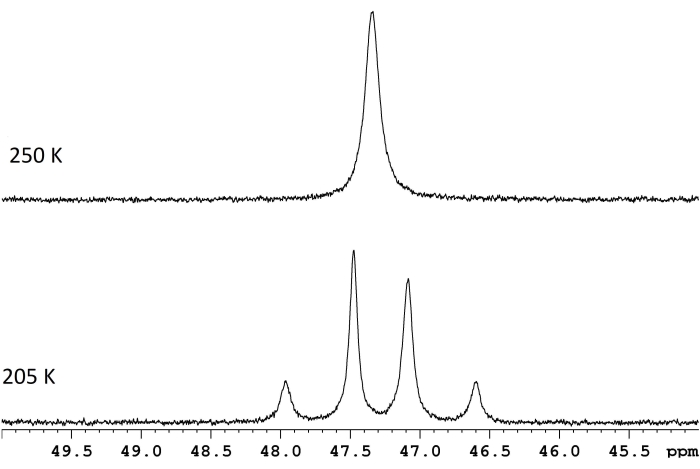
Figure 1: NMR spectra for a d8-toluene solution of ReH5(PPh3)2(sec-butyl amine) at two temperatures. The frequency difference between the two slow exchange doublets (lower trace, 117.8 Hz) and a coalescence temperature of 250 K (upper trace) correspond to an energy barrier (ΔG‡) of 11.8 kcal/mol. Please click here to view a larger version of this figure.
Line shape fitting of dynamic NMR spectra is a common technique that has long been used for the estimation of activation parameters that describe dynamic rearrangements for substances with an activation energy of approximately 5 to 25 kcal/mol2,3,4,5. Determination of the energy barriers to proton exchange between water and amine molecules6, the energy barrier to rotation about the C-N bond in dimethylformamide7, or the general size of organic moieties8 are only a few examples of the many properties that have been assessed through line shape fitting of dynamic NMR spectra. This manuscript demonstrates the use of line shape fitting to characterize the intermolecular and intramolecular dynamic processes that occur for the complex ReH5(PPh3)2(sec-butyl amine). The goals of this and similar line shape fitting NMR experiments are to: 1) characterize all NMR observable intramolecular dynamic atom exchange processes if present, 2) identify and characterize NMR observable intramolecular dynamic atom exchange processes if present, 3) identify correlated intramolecular atom exchanges that occur for, in this example, both hydrogen and phosphorus atoms, and 4) for the example presented here, compare two published models for the dynamic processes that occur in the complex ReH5(PPh3)2(sec-butyl amine).
Eight-coordinate rhenium(V) polyhydride systems are complex dynamic systems in which the ligands participate in multiple dynamic processes and the phosphorus atoms can participate in a single dynamic process that is a second aspect of a hydride ligand exchange process9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,
27,28,29. Eight-coordinate, pseudododecahedral, rhenium(V) polyhydride complexes adopt a molecular geometry (Figure 2), which can be described as a pair of orthogonal trapezoids of ligands17,26. The vertices on the long edges of the trapezoids are commonly labelled as B sites and, in rhenium polyhydride complexes, are usually the sites occupied by neutral two-electron donor ligands such as tertiary phosphines or amine ligands. The vertices on the short edges of the trapezoids are commonly labelled as A sites and are typically occupied by anionic, two-electron donor, hydride ligands. The room temperature NMR spectra of rhenium(V) polyhydride complexes are, typically, deceptively simple due to the several dynamic processes that occur in room temperature solutions.
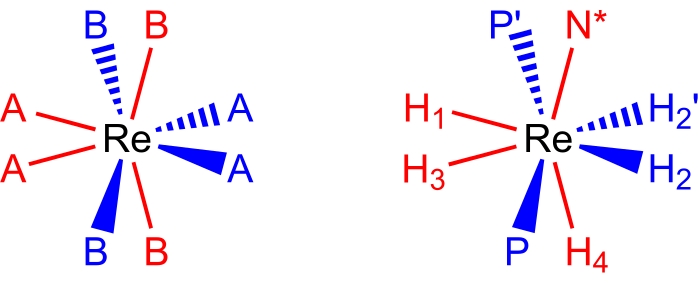
Figure 2: A dodecahedral coordination set (left) and the complex ReH5(PPh3)2(sec-butyl amine) from the same perspective (right). The red-colored sites represent coordination sites that form a vertical trapezoid, and the blue-colored sites represent coordination sites that form a horizontal trapezoid. Please click here to view a larger version of this figure.
Complexes of the form ReH5(PPh3)2(amine) are the most thoroughly studied class of rhenium polyhydride complexes with respect to dynamic processes9,10,12,13,16,30,31. Three dynamic processes (Figure 3) have been identified for ReH5(PPh3)2(amine) complexes: 1) a proton exchange between the sole B site hydride ligand and a proton from a water molecule (adventitious or intentional)9,13, 2) a turnstile exchange of a pair of A site hydride ligands with an adjacent B site hydride ligand9,11,13,30,31, and 3) a steric inversion (or pseudorotation) that manifests itself as a pairwise exchange of the A site hydride ligands and a pairwise movement of the B site atoms to the opposite side of the rhenium center (as depicted in Figure 4)4,5,6,8,26,27. The movement of B site atoms to the opposite side of rhenium is observable by dynamic NMR spectroscopy as: 1) a process that makes the inequivalent 3 and 5 protons of N = pyridine equivalent at room temperature10,30,31, 2) a process that causes the E and Z isomers of N = unsymmetrically substituted aromatic amine ligands to undergo fast exchange at room temperature9,10,13,30,31, or 3) a process that causes a fast exchange of the steric perspectives of a diastereotopic pair of phosphorus atoms with respect to a chiral center located on the amine ligand9,30,31. The previously unreported chiral complex ReH5(PPh3)2(sec-butyl amine) provides an opportunity to generally describe the methods that can be used to identify and characterize the dynamic rearrangements of rhenium polyhydride complexes.
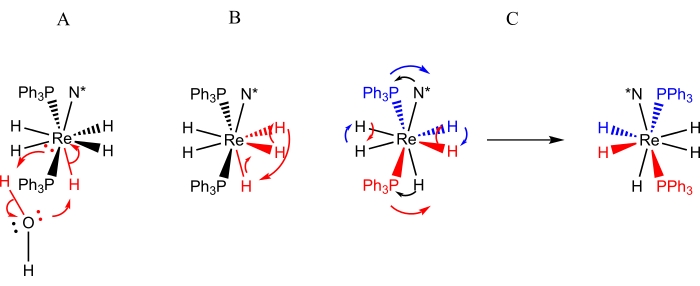
Figure 3: Representations of the dynamic processes that are observed by NMR spectroscopy for solutions of ReH5(PPh3)2(sec-butyl amine). Representation A depicts the exchange of a single proton of adventitious water for the unique B-site hydride ligand. Representation B depicts the turnstile exchange of three adjacent hydride ligands, two of which reside in A site while the third is the unique B site hydride ligand. Representation C depicts both the pairwise exchange of A site hydride ligands as well as the steric inversion of the phosphorus atoms with respect to the chiral amine ligand (N*). It should be noted that the A site hydride ligand pairwise exchange does not require a shift of the A site hydride ligands to the opposite side of the rhenium center. Please click here to view a larger version of this figure.
For chemical systems such as rhenium polyhydride complexes, which exhibit a complex set of dynamic processes, line shape fitting of dynamic NMR spectra is the most used NMR technique to characterize the processes9,11,13,16,21,29. Two-dimensional EXSY9,32 or 2D-ROESY11 are alternative dynamic NMR techniques that can also be used to quantitatively characterize the dynamic processes. Two-dimensional EXSY spectra are typically measured in the slow exchange temperature domain; two-dimensional ROESY spectra are typically measured in the fast exchange temperature domain. Both two-dimensional techniques may require considerable time in the spectrometer for data acquisition, in that each of the techniques is acquiring a much larger data set, at a given temperature, than the one-dimensional data sets needed for line shape fitting analysis. Simple dynamic processes that are well understood, such as the dynamic exchange of the two methyl groups of dimethylformamide, can be readily characterized by any of the three NMR techniques. More complex systems, such as ReH5(PPh3)2(sec-butyl amine), in which individual hydride ligands participate in multiple dynamic processes, or systems that are not necessarily well understood, such as a novel transition metal polyhydride complex which may or may not exchange protons between a hydride ligand and adventitious water, are more easily quantitatively characterized by the line shape fitting NMR method than by the two-dimensional NMR methods. Unlike the two-dimensional NMR methods, the line shape fitting method provides an easily interpretable visualization of the match between a tested model and the experimental data as well as visual evidence of an exchange that moves a hydride ligand beyond the inner coordination sphere of rhenium. Based upon peak heights and peak shapes in slow exchange spectra, even a complex dynamic system such as ReH5(PPh3)2(sec-butyl amine) can lead to an easily tested initial set of exchange models. Additionally, when multiple theoretical models have been reported for a molecular transformation, line shape fitting of dynamic NMR spectra can allow for a visual comparison of each model versus observed spectra.
Beyond the three NMR techniques mentioned above, isotopic substitution NMR experiments involving D2O or HD have been used to qualitatively demonstrate intermolecular exchange of atoms for complex rhenium polyhydride systems, but have not been used for quantitative characterizations9,33,34,35. Theoretical calculations present an additional method for characterizing the dynamic processes of complex dynamic systems30,31,36. Theoretical calculations have the advantage over line shape fitting in that they can be used to differentiate between possibilities that cannot be distinguished by line shape fitting analysis. For example, theoretical calculations have been used to describe an exchange that involves three adjacent hydride ligands on certain rhenium(V) complexes as a turnstile exchange of all three hydride ligands, rather than an alternating pair of pairwise exchanges with each pairwise exchange including a unique hydride ligand and one of two chemically equivalent hydride ligands30,31. The results of theoretical calculations are typically compared to experimentally observed quantitative characterizations from one of the three NMR techniques mentioned above as a check on the validity of the calculated results.
Line shape fitting of dynamic NMR spectra takes advantage of the change in the appearance of NMR spectra that occurs when NMR-active nuclei move between different chemical environments during an NMR measurement. Slow exchange NMR spectra (spectra with independent Lorentzian resonances for each set of exchanging nuclei) occur at temperatures where the frequency difference between resonances for nuclei that exchange is large compared with the rate of exchange of the nuclei37. Fast exchange NMR spectra (spectra with a single Lorentzian resonance for exchanging nuclei) occur at temperatures where the rate of exchange of the nuclei is much greater than the frequency difference between the slow exchange resonances37. Intermediate exchange rates occur for temperatures between the slow exchange temperature domain and the fast exchange temperature domain37. If the fundamental parameters of Larmor frequency, chemical shift of the exchanging nuclei, coupling constants (if any) for the exchanging nuclei, and relative populations of each nucleus type are known, rate constants for putative exchanges between nuclei can be determined by comparing simulated spectra to observed spectra at several intermediate temperatures. Good fits for simulations at several temperatures result in temperature and rate constant data that can be used with the Eyring equation to estimate activation parameters for the putative exchange(s). Results from the method have been found to be both accurate and reproducible.
Protocol
1. Sample preparation
- Preparation of ReH7(PPh3)235
- Combine 0.15 g of sodium borohydride and 0.41 g of ReOCl3(PPh3)2 in a two- or three-necked 100 mL round-bottomed flask fitted with a rubber septum and gas port, or a 100 mL Kjeldahl flask (with a side arm gas port) fitted with a rubber septum (Supplementary Figure 1).
- Add a spin bar to the reaction vessel.
- In a fume hood, use a piece of rubber pressure tubing to connect the gas port of the reaction vessel with one of the stopcocks of a dual glass manifold for vacuum and nitrogen gas. Connect the glass vacuum manifold to a vacuum pump with rubber pressure tubing and connect the glass nitrogen manifold to a regulated nitrogen gas cylinder.
- Connect the exit gas from the nitrogen gas manifold to a stopcock that can be used to direct the vented gas through either a 2 cm column of mineral oil or a 2 cm column of mercury.
- Open the tap on the nitrogen cylinder and adjust the pressure on the flowing gas to 34 pounds per square inch. Vent the nitrogen gas flow through the mercury bubbler.
- Evacuate the gas inside the reaction vessel by adjusting the stopcock on the glass manifold to connect the vessel to the vacuum manifold. Fill the reaction vessel with nitrogen gas by changing the glass manifold stopcock so that it connects the gas manifold with the reaction vessel.
- Repeat steps 1.1.5 and 1.1.6 two more times to completely replace the air in the reaction vessel with nitrogen gas. Chill the flask and its contents in an ice bath.
- Add 8 mL of deoxygenated water and 8 mL of deoxygenated tetrahydrofuran to the solids in the reaction vessel via a syringe. Switch the gas-venting stopcock so that the gas vents through the mineral oil bubbler. Stir the suspension mildly in the ice bath for 15 min. Remove the reaction vessel from the ice bath after the initial 15 min of stirring.
- Allow the mixture to continue stirring for another 45 min. Note the color of the reaction mixture as an indicator of when the reaction has completed. A tan to orange reaction mixture color (Supplementary Figure 1) indicates that the reaction has reached its end point.
- Upon achieving an orange to tan color for the reaction mixture, filter the mixture through a 30 mL medium sintered glass funnel. Wash the recovered solid three times each with 15 mL portions of water, methanol, and ethyl ether. Dry the solid under vacuum to remove any adsorbed solvent.
NOTE: The reaction generally produces between 0.20 g and 0.25 g of product.
- Preparation of ReH5(PPh3)2(sec-butyl amine)
- Weigh 0.070 g of ReH7(PPh3)2 and transfer it into a 50 mL single-necked round-bottomed flask that contains a spin bar. Fit the flask to a condenser equipped with a gas port. Deoxygenate the reaction vessel using the pump and fill method from steps 1.1.3-1.1.7.
- Add a volume of 8 mL of deoxygenated tetrahydrofuran to the reaction vessel via a syringe by cracking the joint between the round-bottomed flask and the condenser. Add a volume of 0.2 mL of sec-butyl amine in a similar fashion. Switch the gas-venting stopcock so that the gas vents to the mineral oil bubbler.
- Heat the reaction mixture to reflux at 65 °C with a heating mantle connected to a variable AC transformer set to 40 on a scale of 0 to 140 for 40 min. Cool the reaction mixture to a temperature that allows for convenient handling of the flask.
- Pour the reaction mixture into 25 mL of methanol in a 125 mL Erlenmeyer flask. Stir the mixture vigorously for 5 min. Add 5 mL of water to induce the formation of a flocculent yellow precipitate.
- Collect the yellow precipitate by vacuum filtration in a sintered glass funnel. Wash the solid with 15 mL of methanol. Dry the solid under vacuum. Following this process, typical product yield is 0.035 g.
2. Acquisition and analysis of NMR spectra
- Measurement of dynamic NMR spectra
- Prepare an NMR sample with approximately 8 mg of the complexReH5(PPh3)2(sec-butyl amine) in about 0.8 mL of d8-toluene. Insert the sample into the instrument.
- Click on the File tab and select New from the choices that appear to open a dialog box that is used to build an NMR experiment.
- Build a 1H experiment by completing the following steps.
- Assign a folder name for the new experiment by completing the Name input box with a unique file name. Assign an experiment number such as 1 for the 1H experiment in the EXPNO box.
- Assign a process number of 1 for the experiment in the PROCNO box. Assign the folder into a directory using the dropdown list for DIR. Identify the solvent that the instrument will lock on from the dropdown Solvent choices.
- Choose the directory that contains the parameters for the 1H experiment from the dropdown list of directories in Experiment Dirs. Select the Proton experiment from the choices in the dropdown Experiment list, and (optional) add a title for the data in the Title fill in box.
- Enter an Eda command in the command line and adjust the parameters as needed to meet the descriptions of the experiment provided in the second paragraph of the Discussion section below.
- Click on the Window tab, select New Window from the list, and repeat Steps 2.1.3.1-2.1.3.8 to prepare a 1H-{31P} experiment using an EXPNO value of 2 to differentiate the experiment from the 1H experiment built previously.
- Click on the Window tab, select New Window from the list, and repeat Steps 2.1.3.1-2.1.3.8 to prepare a 31P-{1H} experiment using an EXPNO value of 3 to differentiate the experiment from the 1H and 1H-{31P} experiments built previously (see Supplementary Table 1 for detailed parameter information).
- Enter a Lock command in the command line and select the d8-toluene choice from the list. Click OK to accept the solvent choice. Enter an Atma command in the command line, if necessary, because of a variable nucleus X-band probe, to minimize reflected energy at the Larmor frequencies for 1H and 31P on the instrument.
- Enter a Ro command on the command line, type a value of 20 into the box, and click on the Start rotation button. Enter a Shim command on the command line. Choose an appropriate autoshim routine such as Topshim from the list of shim routines and click the Start button.
- Enter an Rga command on the command line. Choose the Automatic Receiver Adjust selection and click OK. In turn, measure the three spectra of the sample at room temperature using 64 scans for each spectrum with a Go command on the command line.
- Transform the data from an experiment into a spectrum with an Efp command entered in the command line.
- Adjust the phasing of the spectrum using the following commands.
- Click on the Phase tab followed by a click on the Adjust Phase tab. Hover the cursor over the 0 button on the phasing toolbar and hold the left mouse button down so that the 0 button turns to green.
- With the left mouse button held down, roll the mouse forward or backward until the baseline is flat over the entire spectrum and all of the resonances are displayed as absorbances (peaks rise above the baseline).
- If the baseline cannot be made flat with only the 0 button, adjust the 1 button as described in steps 2.1.10.1 and 2.1.10.2 as well as the 0 button, until the baseline is flat for the entire spectral window.
- Save the phase adjustment with the data by clicking on the Save and Return button on the phasing toolbar.
- Adjust the number of scans for each measurement, as needed, based upon the signal-to-noise ratio in the spectrum, keeping in mind that signal-to-noise typically decreases at lower temperatures due to de-coalescence of the signals into individual resonances (Figure 4).
- Prepare the spectrometer for temperature control as per instructions from the vendor. Enter a flow rate of 200 L/h for the cooling gas and a target temperature of 290 K for the probe. Allow the spectrometer to stabilize at the target temperature for 2 min. Increase the cooling gas flow rate, if needed, to 210 or 220 L/h to stabilize the temperature.
- Shim the sample at 290 K as in Step 2.1.7. Change the file name for each of the previously measured spectra by adding the temperature to the end of the file name (Steps 2.1.2 and 2.1.3.1) and acquire a set of three spectra at 290 K.
- Increase the cooling gas flow rate by ≥ 30 L/h, as needed to stabilize at the next temperature, and decrease the target temperature by 10 K. Allow the spectrometer to stabilize at the next temperature for 2 min and then shim the sample as in Step 2.1.7. Measure the set of three spectra.
- Repeat Steps 2.1.13 and 2.1.14 as needed to acquire spectra down to the lowest temperature desired.
NOTE: A temperature of 200 K is usually sufficient for a complete set of data that is suitable for determining the activation parameters for the dynamic processes of the sample. - Warm the sample back to room temperature in increments of 10 K. Stabilize the temperature for 2 min at each temperature before warming the sample again to prevent damage to the glass liner of the probe.
- Line shape analysis of the measured spectra
- Within the NMR program click on the command bar at the top left of thewindow and select Open from the dropdown menu. Select Open NMR data stored in standard format. Click OK to open the file explorer window for the program.
- Navigate to the folder for the data to be analyzed by line shape fitting. Select the file number that corresponds to the spectrum to be analyzed and click the Display button. The spectrum (if previously processed) or the free induction decay (FID) curve is displayed in the NMR software.
- Process the FID if necessary, by entering an Efp command (exponential multiplication, Fourier transformation, and phase correction) in the command line. Adjust the phase of the spectrum (Step 2.1.10).
- Adjust the baseline of the spectrum; if it is not flat across the entire spectrum then level with the 0-intensity line, as follows.
- Click the Process tab and then click the Baseline tab. Hover the cursor over the A button. Depress the left mouse button and roll the mouse forward or backward to level the red adjustment line with the left (downfield) end of the spectrum.
- If the baseline is still not level with the red adjustment line, repeat the process with the remaining letter buttons until the red adjustment line fits the baseline of the spectrum. Use the save and return button to save the adjustment when the red adjusted baseline matches the actual baseline.
- Select the Analyze tab within the NMR software. Within the analyze options, select the Line Shapes choice followed by the Fit Dynamic NMR Models choice.
- The spectrum is now displayed in the line shape fitting module window. Use the toolbars above the spectrum to adjust how the spectrum is displayed. The window to the left of the spectrum handles the line shape fitting of the spectrum.
- Adjust the spectrum display with the Smooth Zoom Tool so that the portion of the spectrum to be fitted is displayed in the spectrum window. Use the Shift Spectrum Left and Right toolbar button to center a portion of the spectrum in the display window.
- Access the chemical shift window for line shape fitting by selecting the Spectrum tab in the line shape fitting window.
- Click on the Edit Range button. Enter the upper and lower chemical shifts for line shape fitting and click the OK button to accept those limits.
- Start a model for line shape fitting by clicking on the Spin System tab in the line shape fitting window. Click on the Add button to allow for the building of a model spin system.
- Unselect LB (for line broadening) and enter the value for line broadening manually with the mouse and the LB button on the line shape fitting toolbar.
- Add the first nucleus into the model by clicking on the Nucleus tab followed by clicking on the Add button. A set of default values will appear for Nucleus 1. Adjust the chemical shift for Nucleus 1 by entering a value for chemical shift in the Nu(iso) box or with the chemical shift tool on the line shape fitting toolbar.
NOTE: If the selection box is left in the checked form, the chemical shift of this nucleus will be varied to achieve the best fit. Unchecked variables will not be varied in the line fitting process. - Use the Pseudospin box for Nucleus 1 to input the number of equivalent nuclei for Nucleus 1 with each spin 1/2 nucleus equivalent to 0.5 in counting. Enter the sum of the spins into the Pseudospin box in order to account for all equivalent nuclei.
- Use the In Molecule box to accommodate models that require more than a single molecule to participate in a dynamic process. Assign resonances that arise from different molecules to separate molecules using designations such as 1, 2, etc. for different molecules. For resonances that arise from a single molecule, assign 1 for all In Molecule values.
- Add the second and all subsequent nuclei to the model by clicking on the Nucleus tab followed by clicking on the Add button. Include spin-spin coupling between nuclei by either entering the coupling in the appropriate JN box (where N is the nucleus with which the nucleus that is added is coupled, N = 1, 2, …) or by adjusting the Scalar coupling button on the line shape fitting toolbar.
- Begin the process of describing the atom exchanges by clicking on the Reaction tab. Click on the check box if the rate constant for the exchange is to be varied in line shape fitting. Enter the number of nuclei to be exchanged (number with respect to their identifying tabs such as Nucleus 1 and Nucleus 2) into the Exchanges box for the first exchange in the model.
- Describe the exchanges to be tested in the boxes below the Exchanges box. Define the exchanges between Nucleus tabs in the boxes below. A two Nucleus exchange would be entered as Nucleus 1 to Nucleus 2 and Nucleus 2 to Nucleus 1. Ensure that exchanges are cyclic in that if a nucleus is moved from Nucleus 1, another nucleus has to be moved into Nucleus 1.
- Use the Exchange speed button on the line shape fitting toolbar to change the initial value of k in order to iteratively adjust the value of k, even if the check box is selected for the rate constant.
- Add more exchanges to the model by clicking on the Reaction tab followed by clicking on the Add button. Add exchanges to the model as needed. Use the tools on the line shape fitting toolbar to adjust the starting variables, including spectrum intensity to a good match for the spectrum to be fit.
- Begin iterative line shape fitting by clicking on the Start the Spectrum Fit button on the line shape fitting toolbar. Continue iterative fitting until no change is found in the best overlap between spectrum and model or until 1000 iterations are reached. If fitting stops at 1000 iterations, continue further iterations with the Start the Spectrum Fit button. The model spectrum is displayed with the actual spectrum for comparison.
- Record the best fit values from the appropriate tabs. Save the best fit spectrum by clicking on the Spectrum tab in the line shape fitting window followed by clicking on the Save button.
NOTE: The best fit spectrum will be saved in the same folder as was used to collect the data. The best fit spectrum will be distinguished from the original data by being saved with a different processing number that is input when the save occurs. - Save the model used for the line shape fitting by clicking on the Main tab followed by clicking on the Save button. Enter a name for the model.
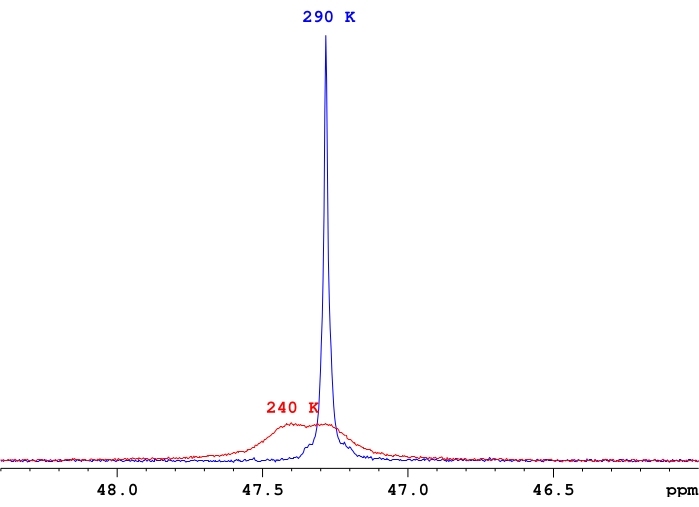
Figure 4: A comparison of 31P-{1H} signal intensities for a single sample of ReH5(PPh3)2(sec-butyl amine) in d8-toluene. A representative demonstration of the difference in signal intensities between a fast exchange single phosphorus resonance and a pair of phosphorus resonances near the coalescence temperature for those resonances. Please click here to view a larger version of this figure.
3. Determination of activation parameters from an Eyring plot 1
- Enter data from line shape fitting for one modelled dynamic process into a spreadsheet with the independent variable entered as 1/T and the dependent variable entered as ln(k/T).
- Insert a scatter plot of the data into the spreadsheet. Add a trend line through the data. Use the slope and intercept of the trend line to solve for ΔH‡ and ΔS‡. The slope of the trend line is -ΔH‡/R while the intercept of the trend line is ΔS‡/R + 23.76.
- Solve for ΔG‡ at a given temperature using the relationship
ΔG‡(T) = ΔH‡ - TΔS‡.
NOTE: For a simple exchange of two nuclei with resonances that coalesce, a check of the values of ΔH‡ and ΔS‡ can be performed by comparing ΔG‡ calculated at the coalescence temperature with the value of ΔG‡ that arises from the slow exchange frequency difference between resonances and the coalescence temperature.
Results
The characterizations of both rhenium polyhydride products described in this manuscript are best accomplished by 1H-{31P} and 31P-{1H} NMR spectroscopy. In a room temperature d6-benzene solution, the hydride ligand resonance of ReH7(PPh3)2 appears as a binomial triplet at δ = -4.2 ppm with 2JPH = 18 Hz by 1H NMR spectroscopy (Supplementary Figure 2). The same d6-benzene...
Discussion
There are four items in the preparation of ReH7(PPh3)2 that can impact the quantity and purity of the material that is produced. First, the use of an ice bath during the first 15 min of the reaction is important to remove heat from the reaction that occurs between sodium borohydride and water. Higher initial temperatures lead to a decreased yield of the ReH7(PPh3)2 product due to formation of the thermal decomposition product Re2H8(PP...
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
The authors thank the Department of Chemistry and Physics and the Creativity and Research Grant Program (Naik, Moehring) at Monmouth University for financial support of this work.
Materials
| Name | Company | Catalog Number | Comments |
| Bruker Avance II 400 MHz NMR spectrometer | Bruker Biospin | The instrument includes a two channel probe (1H and X) with the X channel tunable from 162 MHz to 10 Mhz. The instrument is also VT capable with a dewar and heat exchanger for VT work. | |
| d8-toluene | MilliporeSigma | 434388 | |
| Powerstat variable transformer | Powerstat | ||
| sec-butyl amine | MilliporeSigma | B89000 | |
| Sodium borohydride | MilliporeSigma | 452882 | |
| Tetrahydrofuran | MilliporeSigma | 186562 | |
| Thermowell C3AM 100 mL | Thermowell | ||
| Topspin 3.0 or 4.1.4 with dNMR | Bruker Biospin | Data was acquired with Topspin version 3.0 and data handling was performed on a second computer that was running Topspin version 4.1.4.. | |
| Trichlorooxobis(triphenylphosphine) rhenium(V) | MilliporeSigma | 370193 | |
| Vacuubrand PC3000 vacuum pump with a CVC 3000 controller | Vacuubrand |
References
- Zimmer, K. D., Shoemaker, R., Ruminski, R. R. Synthesis and characterization of a fluxional Re(I) carbonyl complex fac-[Re(CO)3(dpop')Cl] with the nominally tri-dentate ligand dipyrido(2,3-α:3',2'-j)phenazine (dpop). Inorganica Chimica Acta. 359 (5), 1478-1484 (2006).
- McGlinchey, M. J. Symmetry breaking in NMR spectroscopy: the elucidation of hidden molecular rearrangement processes. Symmetry. 6 (3), 622-654 (2014).
- Casarini, D., Luazzi, L., Mazzanti, A. Recent advances in stereodynamics and conformational analysis by dynamic NMR and theoretical calculations. European Journal of Organic Chemistry. 2010 (11), 2035 (2010).
- Palmer, A. G., Williams, J., McDermott, A. Nuclear magnetic resonance studies of biopolymer dynamics. Journal of Physical Chemistry. 100 (31), 13293-13310 (1996).
- Kern, D., Kern, G., Scherer, G., Fischer, G., Drakenberg, T. Kinetic analysis of cyclophilin-catalyzed prolyl cis/trans isomerization by dynamic NMR spectroscopy. Biochemistry. 34 (41), 13594-13602 (1995).
- Menger, F. M., Lynn, J. L. Fast proton transfer at a micelle surface. Journal of the American Chemical Society. 97 (4), 948-949 (1975).
- Pines, A., Rabinovitz, M. A nuclear magnetic resonance total line-shape treatment of internal rotation in dimethylformamide. Tetrahedron Letters. 9 (31), 3529-3532 (1968).
- Mancinelli, M., Bencivenni, G., Pecorari, D., Mazzanti, A. Stereochemistry and recent applications of axially chiral organic molecules. European Journal of Organic Chemistry. 2020 (27), 4070-4086 (2020).
- Streisel, D. J., et al. Fluxionality, substitution, and hydrogen exchange at eight-coordinate rhenium(V) polyhydride centers. Inorganica Chimica Acta. 496 (1), 119028 (2019).
- Jimenez, Y., Strepka, A. M., Borgohain, M. D., Hinojosa, P. A., Moehring, G. A. Ortho-metalation, rotational isomerization, and hydride-hydride coupling at rhenium(V) polyhydride complexes stabilized by aromatic amine ligands. Inorganica Chimica Acta. 362 (9), 3259-3266 (2009).
- Lee, J. C., Yao, W., Crabtree, R. H., Ruegger, H. Fluxionality in [ReH5(PPh3)2(pyridine)]. Inorganic Chemistry. 35 (3), 695-699 (1996).
- Patel, B. P., Kavallieratos, K., Crabtree, R. H. Effects of dihydrogen bonding on fluxionality in ReH5(PPh3)2L. Journal of Organometallic Chemistry. 528 (1), 205-207 (1997).
- Geetha, B., et al. Chiral amine ligands at rhenium(V) pentahydride complexes allow for characterization of an energetically accessible and reversible steric inversion of diastereotopic phosphorus atoms. Inorganica Chimica Acta. 531 (1), 120741 (2022).
- Paulo, A., Ascenso, J., Domingos, A., Galvao, A., Santos, I. Rhenium-(III) and -(V) hydride complexes with modified poly(pyrazolyl)borates. Journal of the Chemical Society, Dalton Transactions. 1999 (8), 1293-1300 (1999).
- Bianchini, C., et al. Synthesis and characterization of rhenium polyhydrides stabilized by the tripodal ligand MeC(CH2PPh2)3. Journal of Organometallic Chemistry. 451 (1), 97-106 (1993).
- Scorzelli, A. G., Macalush, B. E., Naik, D. V., Moehring, G. A. Comparative study of fluxional processes at two different classes of eight-coordinate rhenium(V) polyhydride complexes. Inorganica Chimica Acta. 516 (1), 120120 (2021).
- Luo, X. -. L., Crabtree, R. H. Synthesis and spectroscopic characterization of rhenium complexes ReH5(triphos)] and [ReH6(triphos)]+ [triphos = PPh(CH2CH2PPh2)2]. Journal of the Chemical Society. 1991 (5), 587-590 (1991).
- Kim, Y., Deng, H., Gallucci, J. C., Wojcicki, A. Rhenium polyhydride complexes containing PhP(CH2CH2CH2PCy2)2 (Cyttp): protonation, insertion, and ligand substitution reactions of ReH5(Cyttp) and structural characterization of ReH5(Cyttp) and [ReH4(η2-H2)(Cyttp)]SbF6. Inorganic Chemistry. 35 (24), 7166-7173 (1996).
- Bolano, S., et al. Synthesis, characterization, protonation studies and X-ray crystal structure of ReH5(PPh3)2(PTA) (PTA = 1,3,5-triaza-7-phosphaadamantane). Journal of Organometallic Chemistry. 691 (4), 629-637 (2006).
- Ginsberg, A. P., Abrahams, S. C., Jamieson, P. B. Nonrigid stereochemistry in eight-coordinate pentahydridorhenium complexes. Journal of the American Chemical Society. 95 (14), 4751-4752 (1973).
- Bolano, S., Bravo, J., Garcia-Fontan, S. Mono- and dinuclear rhenium polyhydride complexes bearing the chelating ligand 1,2-bis(dicyclohexylphosphinanyloxy)ethane. European Journal of Inorganic Chemistry. 2004 (24), 4812-4819 (2004).
- Leeaphon, M., Rohl, K., Thomas, R. J., Fanwick, P. E., Walton, R. A. Reactions of the polyhydride complex ReH7(PPh3)2 with quinoline, 2-hydroxyquinoline, and 2-mercaptoquinoline. The preparation and characterization of hydrido complexes of rhenium(V) and chloro complexes of rhenium(III). Inorganic Chemistry. 32 (24), 5562-5568 (1993).
- Mejia, E., Togni, A. Rhenium complexes containing the chiral tridentate ferrocenyl ligand pigiphos. Organometallics. 30 (17), 4765-4770 (2011).
- Moehring, G. A., Walton, R. A. Reactions of heptahydrobis(triphenylphosphine)rhenium with bidentate aromatic heterocycles. Inorganic Chemistry. 26 (17), 2910-2912 (1987).
- Kosanovich, A. J., Reibenspies, J. H., Ozerov, A. V. Complexes of high-valent rhenium supported by the PCP pincer. Organometallics. 35 (4), 513-519 (2016).
- Emge, T. J., Koetzle, T. F., Bruno, J. W., Caulton, K. G. Pentahydridorhenium: crystal and molecular structure of ReH5(PMePh2)3. Inorganic Chemistry. 23 (24), 4012-4017 (1984).
- Costello, M. T., Fanwick, P. E., Green, M. A., Walton, R. A. Reactions of Heptahydridobis(triphenylphosphine)rhenium with 1-(diphenylphosphino)-2-(diphenylarsino)ethane (arphos) and 1,2-bis(diphenylarsino)ethane (dpae). Structural characterization of ReH5(PPh3)2(arphos-As) and ReH5(PPh3)2(dpae-As). Inorganic Chemistry. 30 (4), 861-864 (1991).
- Alvarez, D., Lundquist, E. G., Ziller, J. W., Evans, W. J., Caulton, K. G. Synthesis, structure and applications of transition-metal polyhydride anions. Journal of the American Chemical Society. 111 (22), 8392-8398 (1989).
- Albinati, A., et al. Synthesis, characterization, and interconversion of the rhenium polyhydrides ReH3(η4-NP3)] and [ReH4(η4-NP3)]+ {NP3 = tris[2-(diphenylphosphanyl)ethyl]amine}. European Journal of Inorganic Chemistry. 2002 (6), 1530-1539 (2002).
- Bosque, R., et al. Site preference energetics, fluxionality, and intramolecular M−H···H−N hydrogen bonding in a dodecahedral transition metal polyhydride. Inorganic Chemistry. 36 (24), 5505-5511 (1997).
- Tao, Y., Sou, W., Luo, G. -. G., Kraka, E. Describing polytopal rearrangement processes of octacoordinate structures. I. renewed insights into fluxionality of the rhenium polyhydride complex ReH5(PPh3)2(Pyridine). Inorganic Chemistry. 60 (4), 2492-2502 (2021).
- Beringhelli, T., D'Alfonso, G., Minoja, A. P. Rhenium-platinum mixed metal clusters. Characterization in solution and dynamic behavior of the isomers of [Re3Pt(µ-H3)(CO)14]. An example of a labile metal fragment that undergoes intermolecular exchange. Organometallics. 13 (2), 663-668 (1994).
- Grieco, G., Blacque, O. Solution and solid-state structure of the first NHC-substituted rhenium heptahydrides. European Journal of Inorganic Chemistry. 2019 (34), 3810-3819 (2019).
- Wazio, J. A., Jimenez, V., Soparawalla, S., John, S., Moehring, G. A. Hydrogen exchange of rhenium(VII) heptahydridobis(triphenylphosphine) with water, aniline, methanol, and itself. Inorganica Chimica Acta. 362 (1), 159-165 (2009).
- Chatt, J., Coffey, R. S. Hydrido-complexes of rhenium-containing tertiary phosphines. Journal of the Chemical Society, A. 1969, 1963-1972 (1969).
- Tao, Y., Wang, X., Zou, W., Luo, G. -. G., Kraka, E. Unusual intramolecular motion of ReH92- in K2ReH9 crystal: circle dance and three-arm turnstile mechanisms revealed by computational study. Inorganic Chemistry. 61 (2), 1041-1050 (2022).
- Berger, X., Braun, S. . 200 and More NMR Experiments a Practical Course. , (2004).
- He, G., Chen, J., Sung, H. H. -. Y., Williams, I. D., Jia, G. Substituent effect on reactions of ReH5(PMe2Ph)3 with propargyl alcohols. Inorganica Chimica Acta. 518 (1), 120239 (2021).
- Donnelly, L. J., Parsons, S., Morrison, C. A., Thomas, S. P., Love, J. B. Synthesis and structures of anionic rhenium polyhydride complexes of boron-hydride ligands and their application in catalysis. Chemical Science. 11 (9), 9994-9999 (2020).
- Donnelly, L. J., et al. C-H borylation catalysis of heteroaromatics by a rhenium boryl polyhydride. ACS Catalysis. 11 (12), 7394-7400 (2021).
- Jin, H., et al. CO-enabled rhenium hydride catalyst for directed C(sp2)-H bond alkylation with olefins. Organic Chemistry Frontiers. 2 (4), 378-382 (2015).
- Takaya, H., Ito, M., Murahashi, S. -. I. Rhenium-catalyzed addition of carbonyl compounds to the carbon−nitrogen triple bonds of nitriles: α-C−H activation of carbonyl compounds. Journal of the American Chemical Society. 131 (31), 10824-10825 (2009).
- Carr, S. W., Fowles, E. H., Fontaine, X. L. R., Shaw, B. L. Multihydride complexes of rhenium, osmium or iridium containing monodentate ditertiary phosphine ligands: selective hydrogen-deuterium exchanges of the rhenium multihydrides. Journal of the Chemical Society, Dalton Transactions. 1990 (2), 573-579 (1990).
- Jin, H., et al. Rhenium-catalyzed acceptorless dehydrogenative coupling via dual activation of alcohols and carbonyl compounds. ACS Catalysis. 3 (10), 2195-2198 (2013).
- Loza, M. L., de Gala, S., Crabtree, R. H. Steric crowding in a rhenium polyhydride induced by a chelating disilyl ligand: synthesis, characterization, and reactivity of ReH5(disil)(PPh3)2 (disil = 1,2-Bis(dimethylsilyl)benzene and 1,2-Bis(dimethylsilyl)ethane). Inorganic Chemistry. 33 (22), 5073-5078 (1994).
- Lin, Y., Zhu, X., Xiang, M. Transition metal polyhydrides-catalyzed addition of activated nitriles to aldehydes and ketones via Knoevenagel condensation. Journal of Organometallic Chemistry. 448 (1-2), 215-218 (1993).
- Abdukader, A., Jin, H., Cheng, Y., Zhu, C. Rhenium-catalyzed amination of alcohols by hydrogen transfer process. Tetrahedron Letters. 55 (30), 4172-4174 (2014).
- Lin, Y., Zhou, Y. Selective transfer hydrogenation catalyzed by transition metal polyhydrides. Fenzi Cuihua. 5 (2), 119-124 (1991).
- Green, M. A., Huffman, J. C., Caulton, K. G., Rybak, W. K., Ziolkowski, J. J. Ligand scavenging and catalytic utilization of the phototransient ReH5(PMe2Ph)2. Journal of Organometallic Chemistry. 218 (2), 39-43 (1981).
- Komiya, S., Chigira, T., Suzuki, T., Hirano, M. Polymerization of alkyl methacrylate catalyzed by hydridorhenium complexes. Chemistry Letters. 4 (4), 347-348 (1999).
- Michos, D., Luo, X. L., Faller, J. W., Crabtree, R. H. Tungsten(VI) hexahydride complexes supported by chelating triphosphine ligands: protonation to give η2-dihydrogen complexes and catalytic dehydrogenation of cyclooctane to cyclooctene. Inorganic Chemistry. 32 (8), 1370-1375 (1993).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved