A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Optimized Methods for the Surface Immobilization of Collagens and Collagen Binding Assays
* These authors contributed equally
In This Article
Summary
This work presents an optimized protocol to reproducibly immobilize and quantify type I and III collagen onto microplates, followed by an improved in vitro binding assay protocol to study collagen-compound interactions using a time-resolved fluorescence method. The subsequent step-by-step data analysis and data interpretation are provided.
Abstract
Fibrosis occurs in various tissues as a reparative response to injury or damage. If excessive, however, fibrosis can lead to tissue scarring and organ failure, which is associated with high morbidity and mortality. Collagen is a key driver of fibrosis, with type I and type III collagen being the primary types involved in many fibrotic diseases. Unlike conventional protocols used to immobilize other proteins (e.g., elastin, albumin, fibronectin, etc.), comprehensive protocols to reproducibly immobilize different types of collagens in order to produce stable coatings are not readily available. Immobilizing collagen is surprisingly challenging because multiple experimental conditions may affect the efficiency of immobilization, including the type of collagen, the pH, the temperature, and the type of microplate used. Here, a detailed protocol to reproducibly immobilize and quantify type I and III collagens resulting in stable and reproducible gels/films is provided. Furthermore, this work demonstrates how to perform, analyze, and interpret in vitro time-resolved fluorescence binding studies to investigate the interactions between collagens and candidate collagen-binding compounds (e.g., a peptide conjugated to a metal chelate carrying, for example, europium [Eu(III)]). Such an approach can be universally applied to various biomedical applications, including the field of molecular imaging to develop targeted imaging probes, drug development, cell toxicity studies, cell proliferation studies, and immunoassays.
Introduction
The accumulation of fibrous connective tissue as part of the natural wound-healing process following tissue injury is known as fibrosis. However, if the deposition of fibrous tissue fails to terminate and continues beyond what is needed for tissue repair, then fibrosis becomes excessive1,2. Excessive fibrosis impairs organ physiology and function and could lead to organ damage and potentially organ failure3,4,5. Two main drivers of fibrosis are the extracellular matrix (ECM) proteins collagen type I and type III6. Collagen is a structural protein found in various organs that makes up approximately one-third of the total protein content of the human body1. There are 28 different types of collagens identified by human genome sequencing, and the most abundant are the fibrillar collagens7. The primary fibrillary collagen is type I collagen, which provides the ECM with tensile strength and resistance to deformation8. Type III collagen is a structural component that provides elasticity and colocalizes with type I collagen. It is expressed during embryogenesis and is naturally found in small amounts in adult skin, muscle, and blood vessels9.
In vivo collagen synthesis begins with an intracellular process in which mRNA is transcribed in the nucleus and then moves to the cytoplasm, where it is translated. After translation, the chain formed undergoes post-translational modification in the endoplasmic reticulum, where pro-collagen (the precursor of collagen) is formed. Pro-collagen then travels to the Golgi apparatus for final modification before being excreted to the extracellular space10. Through proteolytic cleavage, pro-collagen is transformed into tropocollagen. This is then cross-linked either via an enzymatic-mediated cross-linking pathway catalyzed by the enzyme lysyl oxidase (LOX) or via a non-enzymatic-mediated cross-linking pathway involving the Maillard reaction11. In vitro protocols to immobilize collagen mainly rely on the ability of collagen to self-assemble. Collagen is extracted from tissues based on its solubility, which largely depends on the extent of cross-linking of individual collagen fibrils7. Fibrillar collagen is dissolved in acetic acid, and fibrils can reform when the pH and temperature are adjusted12. In vitro, the fibrillogenesis of collagen can be viewed as a two-stage process7. The first stage is the nucleation phase, where collagen fibers form dimers and trimer fibrils before they are rearranged to form a triple helical structure. The second phase is the growth phase, where the fibrils start to grow laterally and result in the characteristic D-band formation, which is generally observed by changes in turbidity7. Atomic force microscopy (AFM) studies have also revealed that type I and type III collagen have different characteristics (Table 1)13.
To study the binding interactions between collagen and other compounds, collagen needs to be reproducibly immobilized into the wells of microplates. There are various protocols for immobilizing soluble collagen14,15,16. Commercially available microplates that are pre-coated with collagen are typically used for cell culture. However, pre-coated microplates have a very thin layer of an unknown amount of collagen coated onto the wells, which makes them unsuitable for in vitro binding assays. There are several challenges when immobilizing collagen onto the plate wells. One of the key challenges is choosing a suitable type of microplate, because different types of collagens (e.g., type I and III) have different chemical properties and, therefore, immobilize more stably and effectively depending on the material of the microplate. Another challenge is the experimental conditions of the immobilization protocol, as the process of fibrillogenesis depends on multiple factors, including temperature, pH, the stock concentration of collagen, and the ionic concentration of the buffer7.
For studying the interactions between the collagen (the target) and other compounds (i.e., a targeting peptide), it is also necessary to develop a robust screening assay to investigate the specificity and selectivity of the compound toward the target by measuring the dissociation constant, Kd. The position of the equilibrium of formation of a bimolecular complex between a protein (collagen) and a ligand is expressed in terms of the association constant Ka, whose magnitude is proportional to the binding affinity. However, most commonly, biochemists express affinity relationships in terms of the equilibrium dissociation constant, Kd, of the bimolecular complex, which is defined as Kd = 1/Ka (Kd and is the inverse of Ka).The lower the Kd value, the stronger the binding strength between the protein and the ligand. The advantage of using Kd to compare the binding affinity of different ligands for the same protein (and the other way around) is linked to the fact that the units of Kd for a bimolecular complex are mol/L (i.e., concentration unit). Under most experimental conditions, the Kd value corresponds to the ligand concentration that leads to 50% saturation of the available binding sites on the target at the equilibrium17,18. The dissociation constant is typically extracted by analyzing the receptor fractional occupancy (FO), which is defined as the ratio between the occupied binding sites and total available binding sites, as a function of ligand concentration. This can be done provided that an analytical assay able to distinguish and measure the amount of bound ligand is available.
In vitro ligand binding assays can be performed using various bioanalytical methods, including optical photometry, radioligand methods, inductively coupled plasma mass spectrometry (ICP-MS), and surface plasmon resonance (SPR). Amongst the photometric methods, those based on fluorescence emission typically require the labeling of ligands or proteins with fluorophores to increase the sensitivity and to improve the detection limit of the assay. Chelates of certain lanthanide(III) ions, such as Eu(III), are very attractive as fluorophores as they have large Stokes' shifts, narrow emission bands (providing a good signal-to-noise ratio), limited photobleaching, and long emission lifetimes. Importantly, the latter property enables the use of time-resolved fluorescence (TRF) from Eu(III) fluorophores to abolish background autofluorescence19. In the dissociation-enhanced lanthanide fluorescent immunoassay (DELFIA) version of the Eu(III)-based TRF assay, ligands labeled with a non-luminescent Eu(III)-chelate are incubated with the receptor immobilized onto microplates. The labeled ligand/receptor complex is separated from the unbound ligand, and Eu(III) fluorescence is activated by dissociation of the Eu(III) complex at an acidic pH, followed by re-complexation with a fluorescence-enhancing chelator to form a micelle-embedded, highly fluorescent Eu(III) complex20.
The decomplexation step can be reasonably achieved with chelators, such as diethylenetriamine pentaacetate (DTPA), that show fast decomplexation kinetics. However, Eu(III) complexes with certain macrocyclic chelators, such as DOTA (1,4,7,10-tetraazacyclododecane1,4,7,10-tetraacetic acid) and its monoamide derivatives (DO3AAm), show high thermodynamic stability and very high kinetic inertness. In this case, the decomplexation steps must be accurately optimized to achieve sufficient and reproducible activation of Eu(III)-based TRF21. It is worth noting that lanthanide (Ln(III))-DOTA and Ln(III)-DO3AAm complexes are those most commonly employed as contrast agents for in vivo molecular imaging by magnetic resonance imaging (MRI) techniques22. Thus, the Ln(III)-based TRF assay is the tool of choice to study in vitro the binding affinity of MRI molecular probes with their intended biological targets. Currently, comprehensive and reproducible protocols for immobilizing type I and type III collagen and a reproducible pipeline for performing in vitro binding Eu(III) TRF experiments are lacking. To overcome these limitations, reproducible methods to self-assemble and immobilize type I and type III collagen and generate stable gels and films, respectively, with the sufficient concentration of collagen required for in vitro binding assays, were developed. An optimized protocol for Eu(III) TRF of highly inert Eu(III)-DO3Aam-based complexes is presented. Finally, an optimized in vitro microplate Eu(III) TRF assay to measure the Kd of Eu(III)-labeled ligands toward immobilized type I and type III collagen is demonstrated (Figure 1).
Protocol
NOTE: All product information used for this work is presented in the Table of Materials.
1. Collagen immobilization
NOTE: Ensure each well in the microplate used during the binding assay has adjacent wells free to avoid cross-fluorescence. Carry out this part of the protocol on ice because collagen self-assembles at rising temperatures and pH levels. Perform this procedure in a tissue culture hood and under sterile conditions because the microplates are subsequently incubated in a tissue culture (TC) incubator.
- Immobilization of type I collagen on the 96-well microplates (Figure 2)
Day 1- Prepare a silicone tray with ice. Place the vial containing type I collagen, the cold 10x phosphate-buffered saline (PBS), and the microplates on ice, and spray everything with 70% ethanol. Place the material under the TC hood.
- Neutralize the collagen using equal volumes of type I collagen and 10x PBS (pH 7.4).
- Invert the solution a few times, ensuring that no bubbles form.
- Add 100 µL of the neutralized collagen to every other well and every other row of the microplate, and incubate at 37 °C for 18-20 h to evaporate the collagen to dryness.
Day 2 - Wash the microplates with 100 µL of 1x PBS, pH 7.4, twice to remove any unbound collagen.
- Transfer the microplates into the incubator at 37 °C for another 2 h to dry before using them for further binding experiments.
- Immobilization of collagen type III on the 96-well microplates (Figure 3)
Day 1- Prepare a silicone tray with ice. Place the vial containing type III collagen, the cold 10x PBS, and the microplates on ice, and spray everything with 70% ethanol. Place the material under the TC hood.
- Neutralize the collagen using equal volumes of type III collagen and 10x PBS (pH 7.4).
- Add 70 µL of the neutralized collagen to every other well and every other row of the microplate, and incubate at 37 °C for 2 h by placing the microplate under the tissue culture hood to evaporate the collagen to dryness.
Day 2 - Wash the microplates with 70 µL of 1x PBS, pH 7.4, twice to remove any unbound collagen.
- Transfer the microplates to the incubator for 1 h at 37 °C, and then transfer the microplates to the bench, and allow 1 h to dry before using them in further binding experiments.
2. Assessment of the stability of the immobilized collagen gels/films
- Incubation with PBS for 1 h
NOTE: During the binding experiment, incubate the immobilized collagen with the compound of interest. It is important to investigate the stability of the resulting collagen gel or film. To do this, measure the stability of three conditions: no wash = measures the immobilized collagen directly after incubation; washing = measures the immobilized collagen after washing the plate twice with 100 µL of PBS; and 1 h PBS mimic & wash = measures the immobilized collagen after incubating for 1 h with PBS followed by two washes with PBS. Below, the PBS incubation method is explained.- Add 70 µL of PBS (1x) to each of the wells coated with collagen, and incubate the microplate at room temperature for 1 h.
- Aspirate the excess liquid from each well using a pipette, and wash with PBS (1x) twice before carrying out the protein quantification assay described below.
- Quantification of the amount of immobilized collagen using a bicinchoninic acid assay (BCA)
NOTE: Use the Pierce BCA Protein Assay Kit (Table of Materials) following the manufacturer's instructions. Make respective collagen standards for this assay. The concentration range for collagen I is from 0-3,000 µg/mL and for collagen III from 0-750 µg/mL. In total, make 11 standards per collagen.- Prepare the total volume of working reagent (WR) needed by following the manufacturer's instructions.
- Add 25 µL of each of the collagen standard into the microplate wells (in duplicate). These solutions are used to draw the standard curve.
- Add 200 µL of working reagent solution to each of the wells containing the standards and the wells coated with unknown concentrations of collagen.
- Place the microplate on a plate shaker for 30 s. Cover the microplates, and incubate at 37 °C for 30 min.
- Remove the microplates, and allow to cool at room temperature. Measure the absorbance at 560 nm using a plate reader.
- Plot a calibration curve by plotting the A560 (AU) against the concentration (µg/mL) of the 11 standard solutions, and use the calibration curve to calculate the amount of collagen.
3. Europium(III) TRF ligand binding assay (Figure 1)
NOTE: The compound used is a candidate collagen-binding peptide (CBP) labeled with a single Eu(III)-DO3AAm complex, referred to as Eu(III)-DO3AAm-CBP (Figure 4).
- Incubation of the collagen-coated plates with the Eu(III)-DO3AAm-CBP compound
- Prepare solutions of the Eu(III)-DO3AAm-CBP compound with concentrations ranging between 0.1-15 µM (0.1 µM, 0.5 µM, 1 µM, 3 µM, 5 µM, 7 µM, 10 µM, and 15 µM) in 1x PBS.
- Add 75 µL of each concentration of compound into the collagen-coated wells (Plate A). Perform the experiment in triplicate to calculate the amount of compound that binds to the collagen.
- Use a second uncoated plate (Plate B), and add 75 µL of each compound to the empty wells to calculate the non-specific binding of the compound to the plate. Use triplicates for each concentration.
- Incubate the microplates for 1 h at room temperature.
- Using a pipette, aspirate and discard the excess solution from each well, and wash the wells with 1x PBS twice to remove excess, unbound compound. Perform this step using both the collagen-coated and uncoated microplates.
- To a third uncoated plate (Plate C), add 10 µL of the same range of Eu(III)-DO3AAm-CBP concentrations (in duplicate). Use the fluorescence reading from the Eu(III)-DO3AAm-CBP in solution to make a calibration curve.
NOTE: Do not wash or aspirate the solution from this plate.
- Acid extraction of Europium(III) and time-resolved fluorescence (TRF) readings
NOTE: Please refer to the supplemental information about the preparation and calibration of the volumes of the acidic solution (AS) and the buffering solution (BS). The volumes of the AS and BS required to reproducibly achieve an optimal pH were 54 µL and 46 µL, respectively, in this work. Carry out the following operation on plate A, plate B, and plate C.- Add 54 µL of acidic solution (AS) to each well, and place the plate in the incubator at 37 °C for 90 min, covering the microplates with foil to avoid evaporation. The temperature and incubation time must be carefully controlled to achieve reproducible decomplexation.
- Add 46 µL of buffering solution (BS) to each well, and gently shake the plate for 30 s.
- Add 100 µL of enhancement solution (ES), and shake the plate for 30 s.
- Wait for 30 min before reading the plate using a TRF plate reader. Use the parameters listed in Table 2.
4. Data analysis
- Quantification of the concentration of collagen immobilized on the wells
- Obtain the equation of the calibration curve of the A560 (AU) versus the concentration (µg/mL) of the 11 standard solutions.
- Use the absorbance readings acquired from the wells containing the collagen standards.
- Tabulate the average values from the duplicate wells, and plot the mean absorbance against the known protein (collagen) concentrations (µg/mL) to obtain the equation for the standard curve.
- Use the absorbance values to calculate the mass (µg) and concentration (M) of immobilized collagen.
- Compute the average absorbance values across the three wells that contained immobilized collagen, and record the standard deviation.
- Use the standard curve equation obtained from the collagen standard curve (step 2.2.6) to convert the absorbance measured from the collagen-coated wells into concertation. From this, calculate the concentration of collagen that was immobilized within the experimental wells in µg/mL.
- Convert the concentration calculated in step 4.1.2.2 (µg/mL) first to grams/liter and then, based on the molecular weight of the collagen, into molar (M).
- Finally, calculate the mass of the collagen immobilized in each well by dividing the concentration by the volume of collagen added to the well (100 µL for type I collagen and 70 µL for type III collagen).
- Obtain the equation of the calibration curve of the A560 (AU) versus the concentration (µg/mL) of the 11 standard solutions.
- Calculation of the dissociation constant (Kd) (Figure 4)
- Extract the fluorescence readings.
- Export the fluorescence readings from the plate reader to a spreadsheet.
NOTE: In binding assays, it is important to account for the potential non-specific binding of a compound to the plastic surface of the plates. - Calculate the mean values of triplicate measurements from each compound concentration for the three different plates: the specific binding readings from the coated wells (Plate A), the non-specific binding from the uncoated wells (Plate B) and the total Eu(III)-DO3AAm-CBP in solution in the uncoated wells (Plate C).
- Determine the fluorescence values for the bound compound by subtracting the fluorescence readings of the uncoated wells (Plate B) from that of the coated wells (Plate A).
Equation 1: Determining the bound fluorescence17:
Bound fluorescnece = Specific (coated wells) - Unspecific (uncoated wells) - Generate a calibration curve using the readings from the Eu(III)-labeled compound in solution (Plate C). Plot the fluorescence readings obtained against the concentration of the Eu(III)-labeled compound. Perform a linear regression fit.
- Export the fluorescence readings from the plate reader to a spreadsheet.
- Convert the fluorescence readings to concentrations.
- Convert the readings of the bound fluorescence (step 4.2.3) into concentration using the standard fluorescence curve from the data generated using the compound concentrations in solution (step 4.2.1.4).
NOTE: When comparing the binding properties of one compound toward different target proteins that immobilize at different concentrations, the latter will need to be considered when calculating the amount of compound bound to the target (i.e., bound compound/protein). - Divide the concentration of the bound compound by the concentration of the protein immobilized in the well.
NOTE: For this calculation, use the concentration of immobilized collagen that was calculated after the wells were incubated with PBS for 1 h (so-called PBS mimic experiment; section 2.1 above). This is to account for potential losses of collagen during the incubation step and washing step that will not contribute to the final fluorescence signal. - Plot the data using a scatter plot that has the concentrations of the compound on the x-axis (µM) and the bound compound/protein on the y-axis.
- Convert the readings of the bound fluorescence (step 4.2.3) into concentration using the standard fluorescence curve from the data generated using the compound concentrations in solution (step 4.2.1.4).
- Obtain the Kd values.
- Fit the data acquired in step 4.2.2.3 using two possible binding kinetic models: one-site binding and one-site binding with a hill slope. The equations for each model are shown in Figure 6.
- Choose the model that provides a non-ambiguous fit with the highest R-squared value when fitting the data.
- Exclude the outliers (s) for each set of fluorescence readings per concentration per plate.
- Calculate the final Kd value, and present the data as the mean ± standard deviation of independent experiments.
NOTE: For robust results, perform triplicate measurements within each plate and at least three independent experiments with different microplates.
- Calculate the fractional occupancy (FO).
NOTE: From Equation 2, the concentration of the target is unknown, and, therefore, by using algebra and the Kd, from Equation 3, a workable equation for calculating the fractional occupancy arises in the form of Equation 4.
Equation 2: Definition of fractional occupancy17:
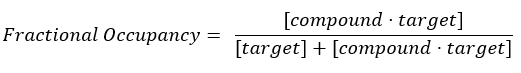
Equation 3: The dissociation constant, Kd, which is the concentration at which the compound occupies 50% of the target at equilibrium17:
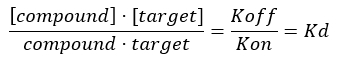
Equation 4: Rearranged equation to calculate the FO equation17:
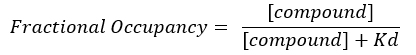
- Calculate the FO using the independent Kd values obtained for each individual plate. Plot the results, mean, and standard deviations of the FO against the concentration of the compound.
- Report the FO with values ranging from 0 to 1 or as a percentage with values ranging from 0%-100%.
- Extract the fluorescence readings.
Results
Assessing the stability and concentration of type I and type III collagen immobilized in gels/films
The quantification of the collagen concentration immobilized per well was carried out using three different conditions: a) in wells without washing with PBS after immobilizing the proteins (no wash); b) in wells with a wash step (twice with PBS) after immobilization to remove any uncoated protein; c) in wells after incubation with PBS for 1 h (PBS mimic experiment). The PBS incubation mimicking step ...
Discussion
This work presents a reproducible method for immobilizing type I and type III collagen. It also demonstrates a protocol for acquiring, analyzing, and interpreting in vitro Eu(III) TRF binding data to characterize the binding properties of a candidate ligand toward type I and III collagen. The protocols for immobilizing type I and type III collagen presented here were developed and optimized considering previously published work on type I and type III collagen fibrillogenesis in vitro13...
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
We are grateful to the following funders for supporting this work: (1) the UK Medical Research Council (MR/N013700/1) and King's College London member of the MRC Doctoral Training Partnership in Biomedical Sciences; (2) BHF program grant RG/20/1/34802; (3) BHF Project grant PG/2019/34897; (4) King's BHF Centre for Research Excellence grant RE/18/2/34213; (5) the ANID Millennium Science Initiative Program - ICN2021_004; and (6) ANID Basal grant FB210024.
Materials
| Name | Company | Catalog Number | Comments |
| 10x PBS | Gibco | 14200075 | Use this to make 1x PBS by diluting in water (1:10) |
| 2M HCL | Made in house and details are in the supporting document | ||
| 2M Sodium hydroxide +2M Glycine | Made in house and details are in the supporting document | ||
| Cell-star 96 well microplate | Greiner Bio-One | 655 160 | |
| DELFIA enhacement solution | Perkin Elmer | 1244-104 | |
| Ice | |||
| Infinite 200 PRO NanoQuant microplate reader | TECAN | ||
| Non-binding (NBS) 96 well microplates | Corning | 3641 | |
| pH electrode Inlab Routine | Mettler Toledo | 51343050 | |
| pH meter (sevenCompact) | Mettler Toledo | ||
| Pierce BCA protein assay kit | Thermofisher | 23227 | |
| Tissue culture incubator (37 °C, 5% CO2) | |||
| Type I bovine collagen, 3 mg/mL | Corning | 354231 | |
| Type III human placenta collagen, 0.99 mg/mL | Advanced Biomatrix | 5021 |
References
- Distler, J. H. W., et al. Review: Frontiers of antifibrotic therapy in systemic sclerosis. Arthritis and Rheumatology. 69 (2), 257-267 (2017).
- Wynn, T. A. Fibrotic disease and the TH1/TH2 paradigm. Nature Reviews Immunology. 4 (8), 583-594 (2004).
- Saha, P., et al. Magnetic resonance T1 relaxation time of venous thrombus is determined by iron processing and predicts susceptibility to lysis. Circulation. 128 (7), 729-736 (2013).
- Mirshahi, M., et al. Defective thrombolysis due to collagen incorporation in fibrin clots. Thrombosis Research. 8, 73-80 (1988).
- Comerota, A. J. The ATTRACT trial: Rationale for early intervention for iliofemoral DVT. Perspectives in Vascular Surgery and Endovascular Therapy. 21 (4), 221-225 (2009).
- Bateman, E. D., Turner-Warwick, M., Adelmann-Grill, B. C. Immunohistochemical study of collagen types in human foetal lung and fibrotic lung disease. Thorax. 36 (9), 645-653 (1981).
- Pawelec, K. M., Best, S. M., Cameron, R. E. Collagen: A network for regenerative medicine. Journal of Materials Chemistry B. 4 (40), 6484-6496 (2016).
- Frantz, C., Stewart, K. M., Weaver, V. M. The extracellular matrix at a glance. Journal of Cell Science. 123 (24), 4195-4200 (2010).
- Copes, F., Pien, N., Van Vlierberghe, S., Boccafoschi, F., Mantovani, D. Collagen-based tissue engineering strategies for vascular medicine. Frontiers in Bioengineering and Biotechnology. 7, 166 (2019).
- Veis, A. The biochemistry of collagen. Annals of Clinical and Laboratory Science. 5 (2), 123-131 (1975).
- Bielajew, B. K., Hu, J. C., Athanasiou, K. A. Collagen: Quantification, biomechanics, and role of minor subtypes in cartilage. Nature Reviews Materials. 5, 730-747 (2020).
- Zhao, Z., et al. Structural and functional plasticity of collagen fibrils. DNA and Cell Biology. 38 (4), 367-373 (2019).
- Eryilmaz, E., Teizer, W., Hwang, W. In vitro analysis of the co-assembly of type-I and type-III collagen. Cellular and Molecular Bioengineering. 10 (1), 41-53 (2017).
- Jagnow, J., Clegg, S. Klebsiella pneumoniae MrkD-mediated biofilm formation on extracellular matrix- and collagen-coated surfaces. Microbiology. 149 (9), 2397-2405 (2003).
- O'Sullivan, D., O'Neill, L., Bourke, P. Direct plasma deposition of collagen on 96-well polystyrene plates for cell culture. ACS Omega. 5 (39), 25069-25076 (2020).
- Caravan, P., et al. Collagen-targeted MRI contrast agent for molecular imaging of fibrosis. Angewandte Chemie - International Edition. 46 (43), 8171-8173 (2007).
- Copeland, R. A. . Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis. , (2000).
- Salahudeen, M. S., Nishtala, P. S. An overview of pharmacodynamic modelling, ligand-binding approach and its application in clinical practice. Saudi Pharmaceutical Journal. 25 (2), 165-175 (2017).
- Bünzli, J. C. G., Piguet, C. Taking advantage of luminescent lanthanide ions. Chemical Society Reviews. 34 (12), 1048-1077 (2005).
- Hemmilii, I. Luminescent lanthanide chelates - A way to more sensitive diagnostic methods. Journal of Alloys and Compounds. 225 (1-2), 480-485 (1995).
- De Silva, C. R., Vagner, J., Lynch, R., Gillies, R. J., Hruby, V. J. Optimization of time-resolved fluorescence assay for detection of europium-tetraazacyclododecyltetraacetic acid-labeled ligand-receptor interactions. Analytical Biochemistry. 398 (1), 15-23 (2010).
- Digilio, G., Lacerda, S., Lavin Plaza, B., Phinikaridou, A. Extracellular matrix targeted MRI probes. Analysis & Sensing. 3 (1), (2022).
- Phinikaridou, A., et al. Tropoelastin: A novel marker for plaque progression and instability. Circulation. Cardiovascular imaging. 11 (8), 007303 (2018).
- Guzaeva, T. V., et al. Protein A used in DELFIA for the determination of specific antibodies. Immunology Letters. 35 (3), 285-289 (1993).
- Nasiri, A. H., Nasiri, H. R. Polymerase assays for lead discovery: An overall review of methodologies and approaches. Analytical Biochemistry. 563, 40-50 (2018).
- Capuana, F., et al. Imaging of dysfunctional elastogenesis in atherosclerosis using an improved gadolinium-based tetrameric MRI probe targeted to tropoelastin. Journal of Medicinal Chemistry. 64 (20), 15250-15261 (2021).
- Drescher, D. G., Drescher, M. J., Ramakrishnan, N. A. Surface plasmon resonance (SPR) analysis of binding interactions of proteins in inner-ear sensory epithelia. Methods in Molecular Biology. 493, 323-343 (2009).
- Murali, S., Rustandi, R. R., Zheng, X., Payne, A., Shang, L. Applications of surface plasmon resonance and biolayer interferometry for virus-ligand binding. Viruses. 14 (4), 717 (2022).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved