Method Article
Two-Step Tag-Free Isolation of Mitochondria for Improved Protein Discovery and Quantification
In This Article
Summary
We present a two-step protocol for high-quality mitochondria isolation that is compatible with protein discovery and quantification at a proteome scale. Our protocol does not require genetic engineering and is thus suitable for studying mitochondria from any primary cells and tissues.
Abstract
Most physiological and disease processes, from central metabolism to immune response to neurodegeneration, involve mitochondria. The mitochondrial proteome is composed of more than 1,000 proteins, and the abundance of each can vary dynamically in response to external stimuli or during disease progression. Here, we describe a protocol for isolating high-quality mitochondria from primary cells and tissues. The two-step procedure comprises (1) mechanical homogenization and differential centrifugation to isolate crude mitochondria, and (2) tag-free immune capture of mitochondria to isolate pure organelles and eliminate contaminants. Mitochondrial proteins from each purification stage are analyzed by quantitative mass spectrometry, and enrichment yields are calculated, allowing the discovery of novel mitochondrial proteins by subtractive proteomics. Our protocol provides a sensitive and comprehensive approach to studying mitochondrial content in cell lines, primary cells, and tissues.
Introduction
Mitochondria are complex and dynamic organelles able to sense and adapt to the metabolic needs of the cell. Central to the complexity of cellular metabolism, mitochondria act as metabolic hubs where carbohydrate, protein, lipid, nucleic acid, and co-factor metabolism reactions converge1. They also serve as signaling organelles for pathways of the innate immune response and in response to changes in ions and reactive oxygen species2,3. To date, around 1,100 proteins have been mapped to mitochondria4,5,6, yet we can assume that many more remain to be discovered, especially those expressed only in certain cell types or transiently under specific environmental conditions. Developing new approaches for quantifying changes in mitochondrial composition in metabolic states of interest will increase our knowledge of these organelles and highlight novel therapeutic avenues for the disorders characterized by mitochondrial dysfunction7.
Currently, different mitochondria isolation protocols are available, with differing yields and levels of purity8. Centrifugation-based approaches are the most popular, due to their simplicity and low cost. Although suitable for most applications, differential centrifugation has the disadvantage of obtaining lower mitochondrial purity and requiring large amounts of starting material when more complex density gradient-based applications are used. In recent years, new methods for mitochondria isolation have emerged, such as tag-based immune capture ("MITO-IP")9 and fluorescence-activated organelle sorting10. Although both procedures can generate samples with high purity, the former requires genetic engineering to tag mitochondria for affinity purification, making the protocols incompatible with primary material from unmodified organisms or human donors. Meanwhile, the latter is dependent on access to flow cytometry and sorting instruments. Combining different isolation methods offers the promise of generating more robust protocols and increased purity.
Here, we present a new protocol for mitochondria isolation based on the combination of two existing methods: (1) differential centrifugation to isolate a crude mitochondrial fraction, and (2) tag-free immune capture of mitochondria with superparamagnetic beads covalently bound to antibodies against translocase of outer mitochondrial membrane 22 (Tomm22)11, a ubiquitous mitochondrial outer-membrane protein (Figure 1). The procedure we describe is compatible with quantitative protein mass spectrometry, and because it is tag-free and does not require genetic manipulation, it can be applied to a wide range of research models, from cell lines to body fluids to whole animal tissues. Furthermore, the use of two steps in the protocol enables the use of subtractive proteomics6,12 for the discovery of novel mitochondrial proteins and the study of their expression.
Protocol
Gloves must be worn at all times and cell culture steps performed under a laminar flow hood. The cells are maintained in a 37 °C incubator with 5% CO2. The research presented in this protocol was approved and performed in compliance with the University of Lausanne and Swiss guidelines for the use of animals.
1. Culture of RAW264.7 macrophage cell line
- Grow the mice macrophage RAW264.7 cells in Dulbecco's modified eagle medium (DMEM) with high glucose and glutamine supplemented with 5% heat-inactivated fetal bovine serum (HI-FBS) and 100 IU/mL penicillin and 100 µg/mL streptomycin (P/S).
NOTE: To isolate mitochondria, a single confluent 15 cm plate (about 70 x 107 RAW264.7 cells) is sufficient. - Maintain the RAW264.7 cells in tissue culture plates. An initial seeding density of 1 x 105 cells/mL leads to a confluent plate in 3 days. Use 25 mL of cell suspension in media for a 15 cm plate. RAW264.7 cells have a high cell division rate and need to be split more frequently than most cell lines.
- Detaching RAW264.7 cells
- Aspirate the media and wash the cells once with phosphate-buffered saline (PBS).
- For a 15 cm plate, add 8 mL of warm RAW dissociation buffer (270 mM potassium chloride, 30 mM sodium citrate dihydrate in H2O, sterile filtered) and incubate the cells at 37 °C for 5 min.
- Add an equivalent volume of media to the plates (1:1 dilution of the dissociation buffer), and pipette to detach and homogenize the cells.
- Transfer the cell suspension to a conical tube and centrifuge the tube at 300 x g for 3 min at room temperature.
- Aspirate the supernatant and resuspend the pellet in an appropriate volume of media (described in step 1.1) for cell counting.
NOTE: Other methods to detach RAW264.7 cells can be used, such as trypsin or a cell scraper. However, these methods are harsher on the cells and may lead to their polarization into M1-like macrophages in the days following detaching.
2. Isolation and culture of bone marrow-derived macrophages (BMDMs)
NOTE: The protocol described here is for a single mouse and can be scaled up for multiple mice. Detailed protocols for BMDM isolation and culture have been described elsewhere13,14.
- Sacrifice an 8-12-week-old C57BL/6 mouse with a high dose of CO2.
NOTE: Either male or female mice can be used. - Spray the mouse with 75% ethanol to sterilize it.
- Dissect and collect the hips, femurs, and tibias from the mouse15.
- To collect the bone marrow from the femurs and tibias, remove the knee joint end of both bones15. Recover bone marrow from the hips by removing the acetabulum.
- Transfer the bones to a 50 mL conical tube with 4 mL of BMDM media kept on ice (DMEM with high glucose and glutamine supplemented with 5% HI-FBS, P/S, and 10 mM HEPES).
NOTE: It is important to keep the bones in the media to avoid drying of the bone marrow during dissection. - Add 4 mL of PBS and 4 mL of warm BMDM media in two different wells of a 6-well plate.
- Transfer the bones and the media from the 50 mL conical tube to an empty well of the 6-well plate.
- Using a pair of forceps, transfer the bones to the PBS well to wash them.
- Transfer the bones to the well containing warm BMDM media.
- Make a hole of 1-2 mm in diameter in the bottom of two 0.5 mL tubes with the forceps and place them in a 1.5 mL tube.
NOTE: It is not necessary to add media to the tube for this quick step. - In each 0.5 mL tube, place a femur, tibia, and hip in such a way that the bone marrow of the exposed bones faces toward the bottom of the tubes.
- Centrifuge the tubes at 13,000 x g for 1 min at room temperature to collect the bone marrow and leftover media through the hole of the 0.5 mL tube and into the 1.5 mL tube. Discard the 0.5 mL tubes with the bones.
- Resuspend the bone marrow pellets in BMDM media and transfer to a 15 mL conical tube.
- Add BMDM media up to 10 mL.
- Place a 40 µm cell strainer on a 50 mL conical tube and filter the bone marrow suspension through it.
- Centrifuge the filtered suspension at 300 x g for 5 min at room temperature to recover the intact cells and remove small debris from the cell suspension.
- Prepare 70 mL of BMDM media supplemented with 50 ng/mL of macrophage colony-stimulating factor (M-CSF).
- Resuspend the pellet in 10 mL of M-CSF-supplemented BMDM media.
- Add 9 mL of M-CSF-supplemented BMDM media to each of the seven 10 cm Petri dishes.
- Plate 1 mL of cells (around 1 x 107 cells) in each of the seven 10 cm Petri dishes.
- Homogenize the cell suspension in each plate by carefully pipetting up and down on the plate and transfer the plates to the incubator.
- After 3 days, add 5 mL of warm BMDM media supplemented with 50 ng/mL M-CSF to each plate.
- After 3 days (day 6, after bone marrow isolation), verify adhesion and differentiation of the BMDM by microscopy.
NOTE: At this point, it is possible to proceed directly to mitochondria isolation (step 3). Alternatively, one can replate the BMDM, which allows treating them with cytokines and small molecules. - Detaching the BMDMs
- Aspirate the media from each plate and add 7 mL of cold PBS supplemented with 5 mM ethylenediaminetetraacetic acid (EDTA).
- Incubate the cells at 4 °C for 7-8 min.
- Detach the BMDMs by carefully pipetting up and down using a 10 mL pipette.
- Pool together the resuspended BMDMs from all seven plates into a single 50 mL conical tube and centrifuge at 300 x g for 3 min at room temperature.
- Aspirate the supernatant and resuspend the cells in 40 mL of warm BMDM media for cell counting.
NOTE: Between 7-9 x 107 BMDMs are obtained per mouse. A minimum of 6 x 107 BMDMs for mitochondria isolation is recommended for proteomics. - If desired, plate and treat the BMDMs according to the experimental goal. If not, proceed directly to step 3.3.
3. Preparation of a crude mitochondrial fraction by differential centrifugation
NOTE: Perform all centrifugation steps at 4 °C. Two centrifuges are required, one with a swing-out rotor and adaptors for conical tubes with a relative centrifugal force of at least 300 x g, the other with a relative centrifugal force of at least 21,000 x g suitable for 1.5 mL tubes. When using adherent cells, use a cell scraper.
- For adherent cells, aspirate the media and add 10 mL of PBS per 15 cm plate.
NOTE: Scraping cells in PBS allows one to wash them at the same time. If the cells are already in the suspension, proceed directly to step 3.3. - Detach the cells using a cell scraper and pool them in a single 50 mL conical tube. Homogenize the cell suspension by pipetting up and down.
NOTE: Cells can be detached using a cell scraper, since this is faster and they will be lysed shortly after. - For each experimental condition, transfer 5% of the cell suspension volume to a 1.5 mL tube and centrifuge it at 300 x g for 5 min at room temperature.
NOTE: When using suspension cells, make sure to wash the cells once with PBS before, to remove possible contaminants from the media such as FBS. - Discard the supernatant and keep the pellet on ice.
NOTE: This will represent the "total cell" fraction for proteomics. - Centrifuge the rest of the samples from step 3.1 or 3.2 at 300 x g for 5 min at room temperature.
- Perform all following steps on ice and using ice-cold buffers.
- Aspirate the supernatant and resuspend the cell pellet in 5 mL of ice-cold mitochondria buffer (MB) (210 mM mannitol, 70 mM sucrose, 10 mM HEPES/NaOH [pH 7.4], and 1 mM EDTA).
- Recover the cells by centrifugation at 300 x g for 5 min at 4 °C.
- Resuspend the cell pellet in 0.5 mL of cold MB and transfer it to a 1.5 mL tube.
NOTE: This procedure yields an approximate cell concentration of 1.5 x 108 BMDM cells/mL or 3 x 108 RAW264.7 cells/mL. - Using a 1 mL syringe fitted with a 25 G needle, homogenize the cell suspension by 30 passages through the needle (Figure 1A).
- Add 1 mL of cold MB to the tube and mix by inversion. Centrifuge the homogenized cell suspension at 2,000 x g for 5 min at 4 °C.
- Transfer 1 mL of the supernatant to a fresh 1.5 mL tube on ice without disturbing the cell pellet. Resuspend the cell pellet and homogenize it again, as in step 3.7.
- Pool the homogenized cell pellet and the supernatant from the two previous steps in a single 1.5 mL tube and centrifuge it at 2,000 x g for 5 min at 4 °C.
NOTE: At this point, the pellet contains mostly nuclei and unbroken cells and is discarded. The supernatant contains cellular debris, cytosol, and organelles, including mitochondria (Figure 1B). - Divide the supernatant among four 1.5 mL tubes.
NOTE: Dividing the supernatant among multiple tubes at this stage improves the removal of contaminants in the following steps. - Add MB to make a final volume of 1 mL in each of the four tubes. Mix by inversion and centrifuge the tubes at 13,000 x g for 10 min at 4 °C.
NOTE: After this step, a pellet with two layers is visible. The bottom, firm, brown pellet contains mitochondria and is kept for further purification (Figure 1C). The upper, loose, white pellet contains other cellular structures and can be discarded. - Perform this step carefully. Remove the supernatant with as much of the white upper pellet as possible. By gently pipetting on it, it is possible to resuspend the white pellet and then discard it, leaving the brown mitochondrial pellet intact.
- Keep one of the four tubes with the mitochondrial pellet on ice. This represents the "crude mitochondria" fraction for proteomics.
- Pool together the other three pellets in a 1.5 mL tube in a final volume of 1 mL of MB.
4. Superparamagnetic antibody-based purification of mitochondria
NOTE: Perform all the following steps in a cold room at 4 °C.
- Transfer 1 mL of the crude mitochondrial preparation from step 3.18 to a 15 mL conical tube and add 7 mL of MB supplemented with 150 mM NaCl (MB + NaCl).
NOTE: The addition of NaCl improves antibody binding and decreases non-specific binding of the contaminants to the beads and to mitochondria. - Add 50 µL of Tomm22 beads to the 8 mL crude mitochondria suspension (Figure 1D) and incubate the tube for 15 min at 4 °C on a rotating wheel at low speed.
NOTE: Tomm22 beads are covalently bound to Tomm22 monoclonal antibodies raised in mouse, coupled to superparamagnetic beads. - Meanwhile, place a column on the magnet.
- Equilibrate the column with 8 mL of MB + NaCl and discard the flowthrough.
- Following the 15 min incubation at 4 °C of the sample with the Tomm22 beads, transfer the sample into the column. Discard the flowthrough.
NOTE: Mitochondria will remain attached to the magnetic beads in the column (Figure 1E). - Wash the column three times with 8 mL of MB + NaCl.
- Remove the column from the magnet and place it in a 15 mL conical tube.
- Elute the mitochondria by adding 1.5 mL of MB + NaCl to the column and apply a plunger immediately to elute the purified mitochondria into the tube.
- Transfer the eluted mitochondria to a 1.5 mL tube and centrifuge it at 21,000 x g for 10 min at 4 °C.
NOTE: A brown pellet will form. This contains the isolated mitochondria and some of the antibody-coupled beads (Figure 1F). - Remove the supernatant carefully from the pellet. The pellet represents the "pure mitochondria" fraction for proteomics. This pellet together with the pellets from steps 3.4 and 3.17 can be stored at -20 °C and are ready for downstream applications.
Results
Three samples with increasing degrees of mitochondrial purity are generated in the present protocol: total cells, crude mitochondria ("mito-crude"), and pure mitochondria ("mito-pure") (Figure 1). We validated the purification of mitochondria from the RAW264.7 macrophage cell line by loading equal protein amounts of each fraction on a gel and immunoblotting, and found that the mitochondrial citrate synthase (Cs) was enriched at each purification step; meanwhile, proteins from the cytosol (GAPDH), the plasma membrane (Na/K ATPase), the nucleus (Lamin B), the lysosomes (Lamp1), and the endoplasmic reticulum (ER) (Pdi) progressively disappeared (Figure 2A). Similar results were obtained using BMDMs. For further validation of the purity and integrity of the isolated mitochondria, electron microscopy on the pure mitochondrial fraction was performed. We observed mitochondria with a classical oval shape and intact cristae surrounded by electron-dense particles corresponding to the antibody-coated beads (Figure 2B). Therefore, it can be concluded that our protocol enriches mitochondria, depletes other cellular components, and maintains mitochondrial structural integrity.
Next, a proteome analysis of each fraction using liquid chromatography coupled to mass spectrometry (LC/MS) was performed. A total of 6,248 proteins in the extract from total cells, 907 of which were previously annotated as mitochondrial in the MitoCarta3.0 inventory5, were identified. After filtering for proteins with a threshold of at least two unique peptides, we calculated an enrichment score for each protein in each sample based on their intensity compared to total cells. We then allocated the proteins to seven major subcellular compartments: mitochondria, ER, lysosomes, Golgi apparatus, cytoskeleton, nucleus, and cytosol, using Gene Ontology (GO)16,17 and MitoCarta3.05 as references. Importantly, an average enrichment for mitochondrial proteins of more than 10-fold and more than 20-fold in the crude and pure mitochondria fractions, respectively, was observed (Figure 2C). In contrast, components of the other six cellular compartments analyzed were depleted during the purification procedure. Of particular note, in the crude mitochondria fraction, we observed a transient enrichment for ER and lysosomal proteins, two classes of contaminant proteins frequently present following differential centrifugation protocols18. This was possibly due to organelle-organelle interactions and similar coefficients of sedimentation, especially for lysosomes, which are highly abundant in macrophages19. While both were mostly depleted after immune capture, we detected a small signal for proteins from the ER-mitochondria contact sites in the mito-pure fraction.
We then directly compared the protein abundance from the total cells and from mito-pure samples and observed two distinct populations, corresponding to mitochondrial and non-mitochondrial proteins (Figure 2D). While the vast majority of MitoCarta proteins clustered together, we found a few (<5%) that clustered with non-MitoCarta proteins. These proteins may represent (1) cytosolic mitochondria-interacting proteins (a novel category annotated in version 3.0 of MitoCarta), (2) dual-localized proteins, or (3) mis-annotated proteins. Conversely, we found a few instances of non-MitoCarta proteins clustering with mitochondrial proteins. While such proteins may represent contaminants of the isolation procedure, they may also represent proteins not previously classified as being present in mitochondria.
To investigate this new class of potential mitochondrial proteins, subtractive proteomics, an approach that has proved useful for the discovery of organellar proteomes, including mitochondria6,12, was used. Subtractive proteomics assumes that mitochondria should become enriched during the purification steps, and contaminants should become depleted6. For example, whereas contaminants may accumulate during differential centrifugation (e.g., due to similar sedimentation properties) or during immune capture (e.g., due to non-specific antibody binding), only bona fide mitochondrial proteins should significantly accumulate in both. It is thus possible to filter out proteins that were found in the pure mitochondria fraction but showed inconsistent patterns of enrichment. In the present example with RAW264.7 cells, by setting a threshold for unique peptides of ≥1 for the mito-crude and mito-pure samples, and using stringent thresholds of enrichment, we were able to refine the list of recovered mitochondrial proteomes from 1,127 proteins initially found in the crude mitochondrial fraction after differential centrifugation, down to 481 proteins following the second round of purification using Tomm22 immunoselection. The reduced number of MitoCarta annotated proteins in the mito-pure fraction reflects the high stringency applied for selection. Interestingly, 70 of the proteins present in the mito-pure fraction were not present in the MitoCarta3.0 inventory (Figure 3A, B). These latter proteins may represent potential novel mitochondrial candidate proteins, which may only be expressed in the RAW264.7 macrophage cell line and in related cells, and which merit further investigation.
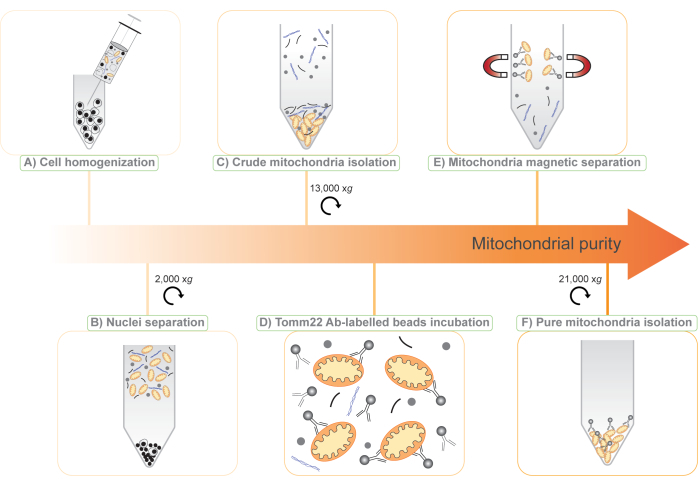
Figure 1: Illustration of the two-step, tag-free mitochondria isolation protocol. (A) A cell suspension is disrupted through a 25 G needle. (B) Nuclei and whole cells are separated by centrifugation at 2,000 x g and the supernatant is saved. (C) Crude mitochondria are isolated by differential centrifugation of the supernatant at 13,000 x g (mito-crude). (D) Crude mitochondria are then incubated with Tomm22 antibodies (Ab) covalently linked to superparamagnetic beads. (E) The mitochondria-Tomm22 antibody-beads complexes are separated from contaminants using magnetic columns and eluted. (F) Pure mitochondria are collected and concentrated by centrifugation (mito-pure). Please click here to view a larger version of this figure.
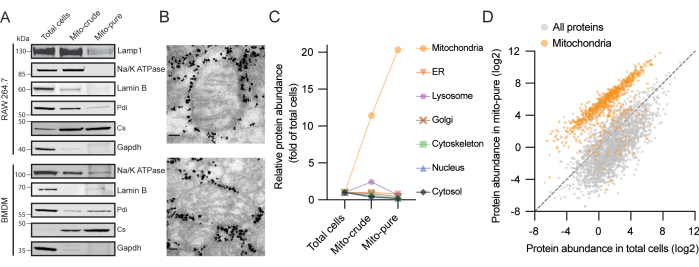
Figure 2. Representative results of mitochondria isolation from two macrophage sources. (A) Protein immunoblot analysis of RAW264.7 (top) and BMDM cells (bottom) using antibodies to mitochondrial citrate synthase (Cs - mitochondria), glyceraldehyde 3-phosphate dehydrogenase (Gapdh - cytosol), sodium-potassium pump (Na/K ATPase - plasma membrane), Lamin B (Lamin B - nucleus), lysosomal-associated membrane protein 1 (Lamp1 - lysosome), and protein disulfide-isomerase (Pdi - ER). (B) Electron microscopy of purified mitochondria from RAW264.7 cells. High density particles surrounding mitochondria correspond to the Tomm22 beads that are carried on with mito-pure samples after elution from the columns. Scale bars: 80 nm. (C) Enrichment scores across total cells, mito-crude, and mito-pure from seven cellular compartments in RAW264.7 cells. MitoCarta3.0 and GO were used for protein annotation and the average scores are represented. Abbreviation: ER = endoplasmic reticulum. (D) Protein abundance values (riBAQ) for proteins in total cells and mito-pure samples from RAW264.7 cells. MitoCarta3.0 proteins are shown in orange. Please click here to view a larger version of this figure.
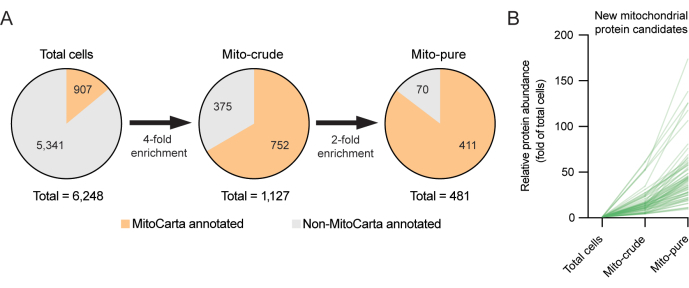
Figure 3. Discovery of novel mitochondrial proteins using subtractive proteomics. (A) Subtractive proteomics strategy for the discovery of novel mitochondrial proteins. High selection thresholds (4x and 2x) are applied to minimize the selection of false positives. (B) Enrichment yields (fold of total cells) of new mitochondrial candidate proteins not previously annotated in the MitoCarta3.0 inventory. Please click here to view a larger version of this figure.
Discussion
We have combined differential centrifugation and immunocapture to achieve an improved purity for mitochondria isolation. Our procedure allows access to primary material for the identification and characterization of novel mitochondrial proteins. The protocol is straightforward and robust, and can be applied to cell lines, primary cells, and tissues without the need for genetic modification. We have validated our protocol by immunoblotting and proteomics analyses on samples taken at different stages throughout the purification procedure.
In comparison to single isolation methods, the combination of enrichment steps of different natures - here, centrifugation and immune labelling - generates a more robust protocol to isolate mitochondria. This is because, while mitochondrial proteins will become enriched in both purifications, it is unlikely that contaminants will also be enriched after both enrichment steps. Although high mitochondrial purity can also be achieved by density gradient ultracentrifugation, this approach requires a large amount of starting material and access to an ultracentrifuge. Lastly, in contrast to recent methods based on tag-based mitochondrial isolation20, our approach does not require genetic modification of the sample, making it suitable for primary material from any source.
Some technical and biological considerations need to be taken into account in the experimental design when applying our protocol. (1) The amount of starting material is critical in order to obtain sufficient material. Inevitably, a small number of mitochondria will be lost during homogenization (step 3.10), as not all cells are lysed, or during the three column washes (step 4.6). While our protocol focuses on purity over yield, the efficiency of mitochondria isolation, and hence their yield, has not been measured or optimized. Using more Tomm22 beads and more columns is expected to increase the yield of mitochondria recovery. At the same time, a thorough optimization of the homogenization step can also lead to improved mitochondrial yield. This protocol and the initial cell numbers we report here for RAW264.7 cells and BMDMs are adequate for proteomics and can be adjusted for other applications. In the case of primary BMDMs, we found that a single mouse was sufficient for one replicate. When necessary, the procedure can be scaled up to isolate BMDMs from multiple animals, which can then be pooled to obtain sufficient material. The cell number can be optimized depending on the cell type, its size, and its mitochondrial content. (2) Tomm22 is expressed on mitochondria from all cell types and tissues21, but its expression level may vary. Therefore, when designing an experiment to compare different conditions, it is important to ensure that the expression levels of Tomm22 are comparable. Furthermore, due to the ubiquitous expression of Tomm22, it is not possible to study cell-type specific mitochondrial proteins within complex tissues. (3) The time necessary to generate pure mitochondria (around 2.5 h) is incompatible with a study of transient events, such as changes in metabolic profiles. In this case, we recommend direct tag-based immune capture9, which also allows studying cell-type specific mitochondria in vivo20. (4) Although studies on isolated mitochondria obtained using Tomm22 antibody-labeled beads alone have shown activity in functional assays11, it remains to be determined whether mitochondria generated with our protocol are compatible with downstream activity-based assays. MitoTracker or tetramethylrhodamine methyl ester perchlorate (TMRM) staining, or respirometry measurements, are potential approaches to quantifying the functionality of isolated mitochondria22. (5) After eluting the "mito-pure" sample from the column, some Tomm22 beads will be present in the pure mitochondria fraction (Figure 2B). While we have observed no interference with trypsin digestion and protein mass spectrometry, the presence of these beads and the immunoglobulins should be taken into consideration in other downstream applications. The Tomm22 antibody is a monoclonal antibody produced in mice23, and therefore it is important to keep in mind that, when using secondary antibodies against mice in immunoblotting, it will generate unspecific bands at the size of the immunoglobulin chains. (6) Complete homogenization of the cell suspension is key to the successful isolation of mitochondria. Here, we use a syringe with a 25 G needle to lyse both RAW264.7 cells and BMDMs. However, depending on the cell type and its size, other mechanical homogenization methods, such as use of a Dounce homogenizer, or more controlled approaches like cell homogenizer devices, may be more suitable. Non-mechanical homogenization methods, such as gentle sonication, can also be considered. Tissue homogenization approaches are further discussed in other studies24,25. (7) Although validation by immunoblotting is the most straightforward and cheaper method, its results might not always correlate with changes at the whole organelle level. That is why we recommend using proteomics to fully validate the enrichment or depletion of mitochondria and other organelles, respectively.
The two-step mitochondria purification protocol described here has allowed us to generate sequential samples with increasing mitochondrial purity, and this has enabled us to discover novel mitochondrial protein candidates through subtractive proteomics12. For our analysis, we use stringent thresholds to select for significantly enriched mitochondrial proteins, and although this may fail to identify some known mitochondrial proteins (Figure 3A), the false-positive rate for new mitochondrial protein discovery is decreased. Nevertheless, it is important to stress that any candidate proteins revealed by our protocol must be validated through orthogonal approaches. We recommend carboxy-terminal GFP-tagging or the use of antibodies against the endogenous protein to validate association with mitochondria either microscopically or by protease protection assays.
The direct application of our method in the case of unmodified cells and tissues offers a powerful tool to investigate how mitochondria change and adapt to their environment in healthy and disease conditions. Application of our protocol to cell lines, animal disease models, human fluids, and even biopsies from surgery may prove to be particularly useful to enhance our understanding of mitochondria and their associated disorders.
Disclosures
The authors have nothing to disclose.
Acknowledgements
We thank Manfredo Quadroni, the Protein Analysis Facility, and the Electron Microscopy Facility at the University of Lausanne for their help. We also thank H.G. Sprenger, K. Maundrell, and members of the Jourdain laboratory for advice and feedback on the manuscript. This work was supported by the Foundation Pierre-Mercier pour la Science, and the Swiss National Science Foundation (project grant 310030_200796).
Materials
| Name | Company | Catalog Number | Comments |
| 1 mL syringe | BD Plastipal | 309628 | |
| 25 G Needle | BD Microlance | 300400 | |
| 40 µm cell strainer | Corning | 352340 | |
| Anti-TOM22 Microbeads, mouse | Miltenyi Biotec | 130-127-693 | |
| Cell scraper | FisherScientific | 11577692 | |
| DMEM, high glucose, GlutaMAX | ThermoFisher | 31966 | |
| Ethylenediaminetetraacetic acid | FisherScientific | BP-120-1 | |
| Fetal bovine serum | Gibco | 10270 | |
| HEPES | BioConcept | 5-31F00-H | |
| LS columns and plungers | Miltenyi Biotec | 130-042-401 | |
| Macrophage colony-stimulating factor | Immunotools | 12343115 | |
| Mannitol | Sigma | M4125 | |
| Sodium chloride | Sigma | 71380 | |
| Sucrose | Sigma | S1888 | |
| Penicillin/Streptomycin | BioConcept | 4-01F00-H | |
| Petri dishes | Corning | BH93B-102 | |
| Phosphate-buffered saline 10X | Eurobio Scientific | CS3PBS01-01 | |
| QuadroMACS Separator | Miltenyi Biotec | 130-090-976 | |
| Vi-CELL BLU Cell Viability Analyzer | Beckman Coulter | C19196 |
References
- Spinelli, J. B., Haigis, M. C. The multifaceted contributions of mitochondria to cellular metabolism. Nature Cell Biology. 20 (7), 745-754 (2018).
- West, A. P., Shadel, G. S., Ghosh, S. Mitochondria in innate immune responses. Nature Reviews Immunology. 11 (6), 389-402 (2011).
- Chakrabarty, R. P., Chandel, N. S. Mitochondria as signaling organelles control mammalian stem cell fate. Cell Stem Cell. 28 (3), 394-408 (2021).
- Morgenstern, M., et al. Quantitative high-confidence human mitochondrial proteome and its dynamics in cellular context. Cell Metabolism. 33 (12), 2464-2483 (2021).
- Rath, S., et al. MitoCarta3.0: an updated mitochondrial proteome now with sub-organelle localization and pathway annotations. Nucleic Acids Research. 49, D1541-D1547 (2021).
- Pagliarini, D. J., et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 134 (1), 112-123 (2008).
- Diaz-Vegas, A., et al. Is mitochondrial dysfunction a common root of noncommunicable chronic diseases. Endocrine Reviews. 41 (3), 005 (2020).
- Bury, A. G., Vincent, A. E., Turnbull, D. M., Actis, P., Hudson, G. Mitochondrial isolation: when size matters. Wellcome Open Research. 5, 226 (2020).
- Chen, W. W., Freinkman, E., Sabatini, D. M. Rapid immunopurification of mitochondria for metabolite profiling and absolute quantification of matrix metabolites. Nature Protocols. 12 (10), 2215-2231 (2017).
- Daniele, J. R., Heydari, K., Arriaga, E. A., Dillin, A. Identification and characterization of mitochondrial subtypes in Caenorhabditis elegans via analysis of individual mitochondria by flow cytometry. Analytical Chemistry. 88 (12), 6309-6316 (2016).
- Franko, A., et al. Efficient isolation of pure and functional mitochondria from mouse tissues using automated tissue disruption and enrichment with anti-TOM22 magnetic beads. PLoS One. 8 (12), e82392 (2013).
- Yates, J. R., Gilchrist, A., Howell, K. E., Bergeron, J. J. M. Proteomics of organelles and large cellular structures. Nature Reviews Molecular Cell Biology. 6 (9), 702-714 (2005).
- Trouplin, V., et al. marrow-derived macrophage production. Journal of Visualized Experiments. (81), e50966 (2013).
- Ying, W., Cheruku, P. S., Bazer, F. W., Safe, S. H., Zhou, B. Investigation of macrophage polarization using bone marrow derived macrophages. Journal of Visualized Experiments. (76), e50323 (2013).
- Toda, G., Yamauchi, T., Kadowaki, T., Ueki, K. Preparation and culture of bone marrow-derived macrophages from mice for functional analysis. STAR Protocols. 2 (1), 100246 (2020).
- Gene Ontology Consortium. The Gene Ontology resource: enriching a GOld mine. Nucleic Acids Research. 49, D325-D334 (2021).
- Ashburner, M., et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nature Genetics. 25 (1), 25-29 (2000).
- Hartwig, S., et al. A critical comparison between two classical and a kit-based method for mitochondria isolation. Proteomics. 9 (11), 3209-3214 (2009).
- Delamarre, L., Pack, M., Chang, H., Mellman, I., Trombetta, E. S. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science. 307 (5715), 1630-1634 (2005).
- Bayraktar, E. C., et al. MITO-Tag mice enable rapid isolation and multimodal profiling of mitochondria from specific cell types in vivo. Proceedings of the National Academy of Sciences. 116 (1), 303-312 (2019).
- Nusinow, D. P., et al. Quantitative proteomics of the cancer cell line encyclopedia. Cell. 180 (2), 387-402 (2020).
- Iuso, A., Repp, B., Biagosch, C., Terrile, C., Prokisch, H. Assessing mitochondrial bioenergetics in isolated mitochondria from various mouse tissues using seahorse XF96 analyzer. Methods in Molecular Biology. 1567, 217-230 (2017).
- Hornig-Do, H. T., et al. Isolation of functional pure mitochondria by superparamagnetic microbeads. Analytical Biochemistry. 389 (1), 1-5 (2009).
- Liao, P. C., Bergamini, C., Fato, R., Pon, L. A., Pallotti, F. Isolation of mitochondria from cells and tissues. Methods in Cell Biology. 155, 3-31 (2020).
- Lanza, I. R., Nair, K. S. Functional assessment of isolated mitochondria in vitro. Methods in Enzymology. 457, 349-372 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved