A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Microinjection of Recombinant RCAS(A) Retrovirus into Embryonic Chicken Lens
In This Article
Summary
This protocol paper describes the methodology of embryonic chicken lens microinjection of an RCAS(A) retrovirus as a tool for studying in situ function and expression of proteins during lens development.
Abstract
Embryonic chicken (Gallus domesticus) is a well-established animal model for the study of lens development and physiology, given its high degree of similarity with the human lens. RCAS(A) is a replication-competent chicken retrovirus that infects dividing cells, which serves as a powerful tool to study the in situ expression and function of wild-type and mutant proteins during lens development by microinjection into the empty lumen of lens vesicle at early developmental stages, restricting its action to surrounding proliferating lens cells. Compared to other approaches, such as transgenic models and ex vivo cultures, the use of an RCAS(A) replication-competent avian retrovirus provides a highly effective, rapid, and customizable system to express exogenous proteins in chick embryos. Specifically, targeted gene transfer can be confined to proliferative lens fiber cells without the need for tissue-specific promoters. In this article, we will briefly overview the steps needed for recombinant retrovirus RCAS(A) preparation, provide a detailed, comprehensive overview of the microinjection procedure, and provide sample results of the technique.
Introduction
The goal of this protocol is to describe the methodology of embryonic chicken lens microinjection of an RCAS(A) (replication-competent avian sarcoma/leukosis retrovirus A). Effective retroviral delivery in an embryonic chicken lens has been demonstrated to be a promising tool for the in vivo study of the molecular mechanism and structure-function of lens proteins in normal lens physiology, pathological conditions, and development. Moreover, this experimental model could be used for the identification of therapeutic targets and drug screening for conditions such as human congenital cataracts. In all, this protocol aims to lay out the necessary steps for the development of a customizable platform for the study of lens proteins.
Embryonic chicks (Gallus domesticus), owing to their similarity in lens structure and function with the human lens, are a well-established animal model for the study of lens development and physiology1,2,3,4. The use of an RCAS(A) replication-competent avian retrovirus has been regarded as a highly effective, rapid, and customizable system to express exogenous proteins in chick embryos. Notably, it has a unique ability to confine the target gene transfer to proliferative lens fiber cells without the need for tissue-specific promoters, using the unique embryonic development time frame in which the presence of empty lens lumen permits in situ RCAS(A) microinjection into the restricted site for the expression of exogenous proteins within proliferative lens fiber cells5,6,7,8.
The chick embryo microinjection procedure, described in-depth here, is based originally partially on the work of Fekete et. al.6 and further developed by Jiang et. al.8 and has been utilized as a means of introducing both viral and nonviral plasmids into the lens of embryonic chicks1,9,10,11,12,13. Overall, the previous work demonstrates the potential of utilizing this methodology to study lens development, differentiation, cellular communication, and disease progression, and for the discovery and testing of therapeutic targets for lens pathological conditions such as cataracts.
Protocol
This study was conducted in compliance with the Animal Welfare Act and the Implementing Animal Welfare Regulations in accordance with the principles of the Guide for the Care and Use of Laboratory Animals. All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio. For an overview of the protocol, see Figure 1; see the Table of Materials for details on all materials, reagents, and instruments used in this protocol.
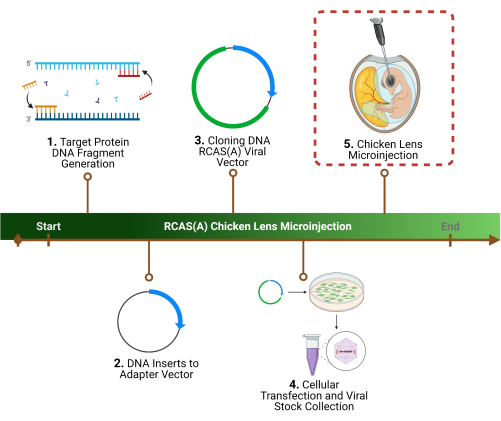
Figure 1: Experimental outline. 1. The first step of the protocol is the determination of a specific target protein(s), the identification of the associated gene sequence(s), and DNA fragment generation. 2. Cloning of the gene sequence(s) into a retroviral vector by initial cloning into an adaptor vector, 3. followed by a viral vector. 4. Preparation of high-titer viral particles using packaging cells to harvest and concentrate. 5. The final step, and the focus of this protocol, is the chicken lens microinjection of the RCAS(A) viral particles into the lens lumen. Please click here to view a larger version of this figure.
1. Preparation of high-titer recombinant retroviruses
- Polymerase chain reaction (PCR) to make target protein DNA fragments
NOTE: This section aims to amplify the DNA sequence corresponding to the target lens protein. For more detail, see7,8.- Design primers for PCR, corresponding to the protein of interest, to make DNA fragments with their carboxyl termini in-frame with or without a FLAG epitope sequence (5'-GACTACAAGGACGACGATGACAAG-3'), a stop codon, and a restriction enzyme site (i.e., EcoRI) present inside the polylinker region of CLa12NCO)) according to the specifications noted in previously published protocols, with sequences for adequate restriction enzymes1,7,8,10,11.
- Perform the PCR reaction10. Combine 100 pmol of sense and antisense of the designed primers with 0.5 µg of connexin cDNA (such as Cx50, Cx43, or chimeric Cx50*43L combinations), to the PCR reaction buffer of choice. Perform 30 cycles each consisting of 94 °C for 1 min, 58 °C for 1 min, and 72 °C for 1 min, followed by a final extension for 10 min at 72 °C.
- Isolate and purify PCR products with a gel purification kit.
- Digest the purified PCR products using restriction enzymes of choice according to the manufacturer's instructions.
- Cloning of DNA inserts into adaptor plasmid
NOTE: Insertion of DNA fragments into an adaptor vector eliminates the need for helper cells/viruses to increase RCAS(A) vector stability14. For more detail, see7,8.- Subclone DNA fragments into an adapter plasmid, Cla12NCO using a sticky-end ligation reaction. Mix the PCR fragments with restriction enzymes and Cla12NCO at a 2-5:1 ratio. Perform ligation and DNA transformation using competent cells.
- Isolate DNA using a DNA isolation kit.
- Sequence the DNA to ensure accuracy.
- Cloning into the RCAS(A) viral vector
NOTE: DNA fragments are inserted into a vehicle (RCAS(A) retrovirus) for the stable transduction of a gene into cells in the developing chick embryo14,15. For more detail, see7,8,15.- Digest the Cla12NCO-DNA fragment of interest-containing vector with ClaI (1 unit per 1 µg of DNA) and gel-isolate the fragments.
- Subclone the DNA fragments into a ClaI-linearized RCAS(A) plasmid. Use the restriction enzyme SalI to distinguish the ClaI site on the RCAS(A) plasmid.
- Confirm the correct orientation of the inserted fragments on the constructs by digestion with ClaI and SalI restriction enzymes.
- Isolate DNA using a DNA isolation kit.
- Determine the DNA concentration.
- Transfection of chick embryonic fibroblast (CEF) cells and RCAS(A) harvest
NOTE: Virus packaging cells, CEFs, are used to obtain/harvest cell culture media containing viral particles15,16. For more detail, see7,8,15.- Transfect CEF cells with recombinant retroviral DNA constructs using the transfection agent according to the manufacturer's instructions.
- Perform western blotting of the transfected cells to examine their expression of the protein of interest.
- When the transfected cells reach confluency, begin collecting the supernatant medium, processing/filtering it appropriately (to remove any cellular debris using a 0.22µm filter), and store at -80 °C until ready for concentration.
- Concentration and titering of viral stocks
NOTE: The pellet and large volumes of media derived from the cells containing the viral particles must be filtered. Additionally, a viral titer assay must be performed to establish the strength of the virus against the host cells. For more detail, see6,7,8,15.- Thaw the culture media containing the virions.
- Spin the culture media at 72,000 × g for 2 h at 4 °C.
- Decant the supernatant and resuspend the viral pellet with the residual (~50 µL) medium and store at -80 °C until use, in ~10 µL viral stocks.
- For titering, using either QT-6 or CEF cells, transfect the cells with a serial dilution of the viral stocks.
- Post confluency, fix the cells using 4% formaldehyde.
- Immunostain for viral gag proteins or process for alkaline phosphatase histochemically to obtain the titer, defined as (colony-forming units) CFU per milliliter (CFU/mL).
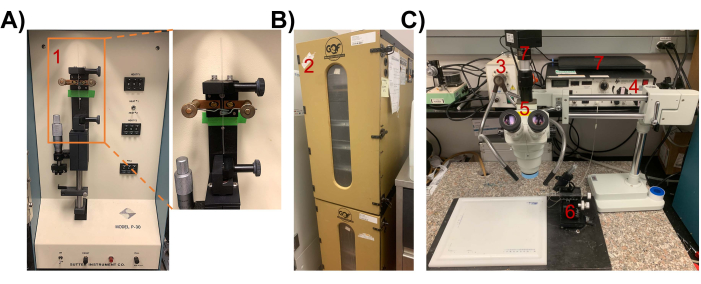
Figure 2: Instruments and setup for chick lens microinjection. (A) P-30 manual vertical microelectrode micropipette puller. (1) inset showing a glass micropipette being pulled. (B) (2) Egg Incubator. Incubate chicken egg for ~65-68 h in a 37 °C incubator to reach stage 18 (with a sealed, empty central lumen) for injection of retrovirus. (C) Microinjection setup. (3) lighting equipment, (4) Pico-Injector, (5) dissecting microscope, (6) Drummond micromanipulator, (7) computer/camera for visualization. Please click here to view a larger version of this figure.
2. Chick lens microinjection
- Preparation of supplies
- Preparation of glass micropipettes for microinjection17
NOTE: Glass capillaries are used to make glass micropipettes with a tip point and an outer diameter (OD) of ~11 µm for microinjection. The setup is shown in Figure 2A.- Attach the borosilicate glass capillary to the rubber cushioned clips in the manual vertical micropipette puller.
- Set heat temperature (HEAT 1) to 950 °C and perform prepulling to obtain a thinner and softer glass capillary.
- Set heat temperature (HEAT 2) to 790 °C and perform a secondary pulling to produce final micropipettes.
- With a micropipette grinder, sharpen the tip opening to an OD of ~11 µm.
- For future experiments, keep the glass micropipettes in a sponge clamping pad inside a glass jar.
- Incubation of fertilized eggs until development stage 18
NOTE: During lens development, at ~65-68 h of embryonic development, the lens separates from the ectoderm and forms a sealed vesicle with a central lumen; injection into this empty lens lumen primes for restricted expression to proliferative lens cells7,8,18.- Incubate fertilized chicken eggs for ~65-68 h in a 37 °C humidified rocking incubator (Figure 2B).
- Preparation of glass micropipettes for microinjection17
- Opening of the chicken egg
NOTE: This step describes the identification and exposure of the embryo for microinjection. For more detail, see7,8.- Wipe down the work area thoroughly with 70% ethanol.
- Remove the eggs from the incubator, spray them down heavily with 70% ethanol, and let them air-dry.
- Place the egg, with the larger end of the egg facing upwards, on an egg holder.
- Using a pair of sharp-toothed forceps, produce a hole of ~2 cm diameter on the larger end of the egg by carefully tapping on the eggshell and removing fragments of the eggshell (Figure 3A).
- Using a dissecting microscope, locate the embryo.
- Once the embryo and chick lens are located, use the dissecting microscope and fine dissecting forceps and scissors to cut off the amnionic membrane immediately covering the top of the embryo (Figure 3B).
NOTE: Do not cut too widely (only enough to expose the embryo ~0.5 cm x 0.5 cm), or else the embryos might sink into the yolk mass; also avoid touching blood vessels, if possible. - Cover the opening of the egg with a 60 mm Petri dish in preparation for the next steps.
- Microinjection of concentrated viral stock
NOTE: Viral particles containing target protein DNA fragments for transduction are inserted into cells inside the lens lumen. The setup is shown in Figure 2C. For more detail, see6,7,8.- Thaw the viral stock stocks on ice.
- For visualization of the viral stock during injection, dilute 1 µL of 10x Fast green into one vial of viral stock containing 10 µL.
- To ensure there are no large chunks of undissolved material that could clog the glass micropipette, centrifuge the solutions for 10 s at 10,000 × g at 4 °C to pellet "large chunks" and transfer the supernatants into new tubes, all while keeping the solutions on ice.
- On a 35 mm x 10 mm culture dish, place a stretched laboratory parafilm and add 1 µL of sterile saline (PBS) on top of the film (non-sterilized, with the covered side considered "clean").
- Connect a prepared glass micropipette to an automatic pico-injector.
- Press the fill mode to fill the sterile saline (PBS) into a glass micropipette, and then use the inject mode to test the allocated 1 µL of liquid.
- Place a second stretched laboratory parafilm on the surface of a new 35 mm x 10 mm cell culture dish and add 1 µL of Fast green-stained viral stocks on top of the film.
- Lower the tip of the micropipette into the viral stock and press the fill-model to fill the glass micropipette with ~1 µL of viral stock.
NOTE: To avoid drying out, put the tip of the filled micropipette in sterile saline (PBS) whenever there is a pause in the procedure. - Lower the filled glass micropipette, connected to an automatic injector, into the target region of the embryo lens lumen with the assistance of a dissecting microscope.
- Adjust the light source to ensure clear visualization of the outline of the lens vesicle.
NOTE: The lumen of the right lens (facing up) is typically used for injection and the left (facing down) is kept intact as a contralateral control. - Once sure that the placement of the micropipette is within the correct location inside the lens lumen, inject 5-40 nL of the viral stock (Figure 3C).
NOTE: Practice is very necessary for optimal injection placement. - Wait for ~45 s and then gently remove the glass micropipette.
- Check for the successful microinjection into the lens lumen using the dissecting microscope by examining Fast Green dye that should stay in the empty lumen of the lens without any leakage.
- After the injection procedure, seal the eggshell opening with scotch tape.
- Return the embryo to the 37 °C humidified incubator, without any rotating motion, until the desired embryonic age has been reached for lens dissection.
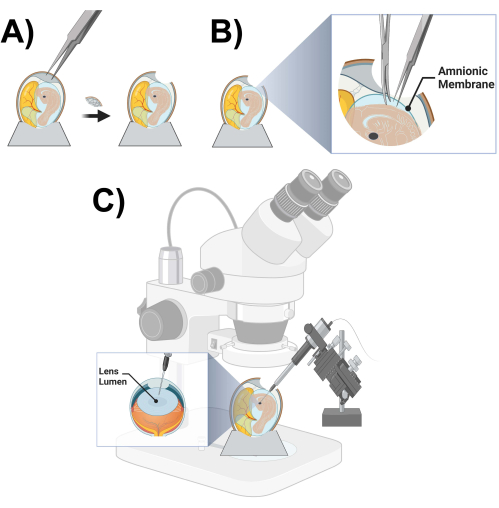
Figure 3: Microinjection chick prep and schematic. (A) Opening of a chicken egg. (B) Cutting of the amniotic membrane. (C) Chicken lens lumen microinjection schematic. Please click here to view a larger version of this figure.
Results
After the determination of a specific target protein(s) and the identification of the associated gene sequence(s), the overall experimental approach involves the cloning of the gene sequence(s) into a retroviral RCAS(A) vector by the initial cloning into an adaptor vector, followed by a viral vector. Second, high-titer viral particles are prepared using packaging cells to harvest and concentrate the virions. These first two major steps have been largely described and representative results presented elsewhere
Discussion
This experimental model offers the opportunity to express the protein(s) of interest in the intact lens leading to the study of the functional relevance of these proteins in lens structure and function. The embryonic chick microinjection model is based partially on the work of Fekete et. al.6 and was further developed by Jiang et. al.8 and has been utilized as a means of inserting both viral plasmids and agents such as agonists, small interfering RNA (siRNA), and peptides i...
Disclosures
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) Grants: RO1 EY012085 (to J.X.J) and F32DK134051 (to F.M.A), and Welch Foundation grant: AQ-1507 (to J.X.J.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The figures were partially created with Biorender.com.
Materials
| Name | Company | Catalog Number | Comments |
| 0.22 µm Filter | Corning | 431118 | For removing cellular debris from media |
| 35 mm x 10 mm Culture Dish | FisherScientific | 50-202-030 | For using during microinjection |
| Centrifuge | Fisherbrand | 13-100-676 | Spinning down solution |
| Constructs | GENEWIZ | - | For generation of constructs |
| Dissecting microscope | AmScope | SM-4TZ-144A | Visualization of lens for microinjection |
| DNA PCR primers | Integrated DNA Technologies | - | Generation of primers: Intracellular loop (IL)-deleted Cx50 (residues 1–97 and 149–400) as well as the Cla12NCO vector were obtained with the following pair of primers: sense, CTCCTGAGAACCTACATCCT; antisense, CACCGCATGCCCAAAGTACAC ILs of Cx43 (residues 98–150) and Cx46 (residues 98–166) were obtained with the following pairs of primers: sense, TACGTGATGAGGAAAGAAGAG; antisense, TCCTCCACGCATCTTTACCTTG; sense, CACATTGTACGCATGGAAGAG; antisense, AGCACCTCCC AT ACGGATTC, respectively Cla12NCO-Cx43 construct template was obtained with the following pair of primers: sense, CTGCTTCGTACTTACATCATC; antisense, GAACAC GTGCGCCAGGTAC ILs of Cx50 (residues 98–148) or Cx46 (residues 98–166) were cloned by using Cla12NCO-Cx50 and Cla12NCO-Cx46 constructs as the templates with the following pair of primers: sense, CACCATGTCCGCATGGAGGAGA; antisense, GGTCCCC TC CAGGCGAAAC; sense, CACATTGTACGCATGGAAGAG; antisense, AGCACCTCCCATACGGATTC, respectively |
| Drummond Nanoject II Automatic Nanoliter Injector | Drummond Scientific | 3-000-204 | Microinjection Pipet |
| Dual Gooseneck Lights Microscope Illuminator | AmScope | LED-50WY | Lighting for visualization |
| Dulbecco’s Modified Eagle Medium (DMEM) | Invitrogen | For cell culture | |
| Egg Holder | - | - | Homemade styrofoam rings with 2-inch diameter and one-half inch height |
| Egg Incubator | GQF Manufacturing Company Inc. | 1502 | For incubation of fertilized eggs |
| Fast Green | Fisher scientific | F99-10 | For visualization of viral stock injection |
| Fertilized white leghorn chicken eggs | Texas A&M University | N/A | Animal model of choice for microinjection (https://posc.tamu.edu/fertile-egg-orders/) |
| Fetal Bovine Serum (FBS) | Hyclone Laboratories | For cell culture | |
| Fluorescein-conjugated anti-mouse IgG | Jackson ImmunoResearch | 115-095-003 | For anti-FLAG 1:500 |
| Forceps | FisherScientific | 22-327379 | For moving things around and isolation |
| Glass capillaries | Sutter Instruments | B100-75-10 | Glass micropipette for microinjection (O.D. 1.0 mm, I.D. 0.75 mm, 10 cm length) |
| Lipofectamine | Invitrogen | L3000001 | For transfection |
| Manual vertical micropipette puller | Sutter Instruments | P-30 | To obtain glass micropipette of the correct size |
| Microcentrifuge Tubes | FisherScientific | 02-682-004 | Dissolving solution |
| Microscope | Keyence | BZ-X710 | For imaging staining |
| Parafilm | FisherScientific | 03-448-254 | Placing solution |
| Penicillin/Streptomycin | Invitrogen | For cell culture | |
| Pico-Injector | Harvard Apparatus | PLI-100 | For delivering small liquid volumes precisely through micropipettes by applying a regulated pressure for a digitally set period of time |
| rabbit anti-chick AQP0 | Self generated | - | Jiang JX, White TW, Goodenough DA, Paul DL. Molecular cloning and functional characterization of chick lens fiber connexin 45.6. Mol Biol Cell. 1994 Mar;5(3):363-73. doi: 10.1091/mbc.5.3.363. |
| rabbit anti-FLAG antibody | Rockland Immunichemicals | 600-401-383 | For staining FLAG |
| Rhodamine-conjugated anti-rabbit IgG | Jackson ImmunoResearch | 111-295-003 | For anti-AQP0 1:500 |
| Sponge clamping pad | Sutter Instruments | BX10 | For storage of glass micropipette |
References
- Li, Z., Gu, S., Quan, Y., Varadaraj, K., Jiang, J. X. Development of a potent embryonic chick lens model for studying congenital cataracts in vivo. Communications Biology. 4 (1), 325 (2021).
- Chen, Y., et al. γ-Crystallins of the chicken lens: remnants of an ancient vertebrate gene family in birds. The FEBS Journal. 283 (8), 1516-1530 (2016).
- Coulombre, A. J., Coulombre, J. L. Lens development. I. Role of the lens in eye growth. Journal of Experimental Zoology. 156 (1), 39-47 (1964).
- McKeehan, M. S. Induction of portions of the chick lens without contact with the optic cup. The Anatomical Record. 132 (3), 297-305 (1958).
- Kothlow, S., Schenk-Weibhauser, K., Ratcliffe, M. J., Kaspers, B. Prolonged effect of BAFF on chicken B cell development revealed by RCAS retroviral gene transfer in vivo. Molecular immunology. 47 (7-8), 1619-1628 (2010).
- Fekete, D. M., Cepko, C. L. Replication-competent retroviral vectors encoding alkaline phosphatase reveal spatial restriction of viral gene expression/transduction in the chick embryo. Molecular and Cellular Biology. 13 (4), 2604-2613 (1993).
- Jiang, J. X. Use of retroviruses to express connexins. Methods in Molecular Biology. , 159-174 (2001).
- Jiang, J. X., Goodenough, D. A. Retroviral expression of connexins in embryonic chick lens. Investigative Ophthalmology & Visual Science. 39 (3), 537-543 (1998).
- Shestopalov, V. I., Bassnett, S. Expression of autofluorescent proteins reveals a novel protein permeable pathway between cells in the lens core. Journal of Cell Science. 113 (11), 1913-1921 (2000).
- Liu, J., Xu, J., Gu, S., Nicholson, B. J., Jiang, J. X. Aquaporin 0 enhances gap junction coupling via its cell adhesion function and interaction with connexin 50. Journal of Cell Science. 124 (2), 198-206 (2011).
- Li, Z., et al. The second extracellular domain of connexin 50 is important for in cell adhesion, lens differentiation, and adhesion molecule expression. Journal of Biological Chemistry. 299 (3), 102965 (2023).
- Shestopalov, V. I., Bassnett, S. Exogenous gene expression and protein targeting in lens fiber cells. Investigative Ophthalmology & Visual Science. 40 (7), 1435-1443 (1999).
- Shestopalov, V. I., Bassnett, S. Three-dimensional organization of primary lens fiber cells. Investigative Ophthalmology & Visual Science. 41 (3), 859-863 (2000).
- Hughes, S. H., Greenhouse, J. J., Petropoulos, C. J., Sutrave, P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. Journal of Virology. 61 (10), 3004-3012 (1987).
- Yan, R. T., Wang, S. Z. Production of high-titer RCAS retrovirus. Methods in Molecular Biology. 884, 193-199 (2012).
- Kingston, R. E. Introduction of DNA into mammalian cells. Current Protocols in Molecular Biology. 64 (1), 1-95 (2003).
- Li, Y., et al. Studying macrophage activation in immune-privileged lens through CSF-1 protein intravitreal injection in mouse model. STAR Protocols. 3 (1), 101060 (2022).
- Hamburger, V., Hamilton, H. L. A series of normal stages in the development of the chick embryo. Developmental Dynamics. 195 (4), 231-272 (1992).
- Hallagan, J. B., Allen, D. C., Borzelleca, J. F. The safety and regulatory status of food, drug and cosmetics colour additives exempt from certification. Food and Chemical Toxicology. 33 (6), 515-528 (1995).
- Okada, T. S., Eguchi, G., Takeichi, M. The expression of differentiation by chicken lens epithelium in in vitro cell culture. Development, Growth & Differentiation. 13 (4), 323-336 (1971).
- Menko, A. S., Klukas, K. A., Johnson, R. G. Chicken embryo lens cultures mimic differentiation in the lens. Developmental Biology. 103 (1), 129-141 (1984).
- Parreno, J., et al. Methodologies to unlock the molecular expression and cellular structure of ocular lens epithelial cells. Frontiers in Cell and Developmental Biology. 10, 983178 (2022).
- Edwards, A., Gupta, J. D., Harley, J. D. Photomicrographic evaluation of drug-induced cataracts in cultured embryonic chick lens. Experimental Eye Research. 15 (4), 495-498 (1973).
- Musil, L. S. Primary cultures of embryonic chick lens cells as a model system to study lens gap junctions and fiber cell differentiation. Journal of Membrane Biology. 245 (7), 357-368 (2012).
- West-Mays, J. A., Pino, G., Lovicu, F. J. Development and use of the lens epithelial explant system to study lens differentiation and cataractogenesis. Progress in Retinal and Eye Research. 29 (2), 135-143 (2010).
- Upreti, A., et al. Lens epithelial explants treated with vitreous humor undergo alterations in chromatin landscape with concurrent activation of genes associated with fiber cell differentiation and innate immune response. Cells. 12 (3), 501 (2023).
- Walker, J. L., Wolff, I. M., Zhang, L., Menko, A. S. Activation of SRC kinases signals induction of posterior capsule opacification. Investigative Ophthalmology & Visual Science. 48 (5), 2214-2223 (2007).
- Briskin, M. J., et al. Heritable retroviral transgenes are highly expressed in chickens. Proceedings of the National Academy of Sciences of the United States of America. 88 (5), 1736-1740 (1991).
- Hughes, S. H. The RCAS vector system. Folia Biologica. 50 (3-4), 107-119 (2004).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved