A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Purification of the Sarco-Endoplasmic Reticulum Ca2+-ATPase from Rabbit Muscle
In This Article
Summary
This protocol describes an improved SERCA purification method, which includes the disaccharide trehalose in the final centrifugation step. This carbohydrate stabilizes proteins under harsh conditions. The purified SERCA was catalytically active and displayed high purity, making it suitable for structural and functional studies.
Abstract
Some P-type ATPases, such as sarco/endoplasmic reticulum Ca2+-ATPase (SERCA), are inherently labile membrane proteins that require specific physicochemical conditions during purification to obtain them with high purity and structural quality and in a catalytically active form. The disaccharide trehalose is a compatible solute that is synthesized and accumulated in high concentrations in the yeast cytoplasm to stabilize the membranes and proteins. The use of trehalose as an additive in the protocol for the purification of plasma membrane H+-ATPase results in a high-quality preparation, the hexameric structure of which is shown by biochemical analytical methods. Trehalose can, therefore, be used as a stabilizing additive for the purification of membrane proteins (P-ATPases). This protocol describes the modification of the classical protocol for SERCA purification by subjecting SERCA to centrifugation on a trehalose concentration gradient. The inclusion of this carbohydrate led to the purification of SERCA in a catalytically active form with high purity and, importantly, in a stable form. Partial biochemical characterization of the purified SERCA (SDS-PAGE, enzyme kinetics, FITC labeling, circular dichroism spectroscopy) showed that the enzyme is suitable for functional and structural studies. The use of trehalose in the purification protocol of P-type ATPases and other labile membrane (and cytosolic) proteins is suggested.
Introduction
Membrane proteins/enzymes are essential biological components of cells as they play critical roles in various processes1,2,3. Some of the functions may include transport of ions and molecules in and out of the cell/internal compartments (either active/passive), cell-cell recognition, intercellular binding, anchorage/attachment, and sensing of the external environment through integration with the signal transduction machinery under normal and harsh physical and chemical conditions (high salt, low water, high temperature, drug resistance, etc.)3 Therefore, the determination of the three-dimensional (3D) structure of membrane proteins and/or enzymes has become of great importance for both basic and applied research4,7,6. Importantly, membrane proteins/enzymes have been extensively used as targets for drug discovery (whether natural or by design)7,8,9. That is, membrane proteins have an inherent importance in health9,10,11.
The hydrophobic character of membrane proteins/enzymes is the most technically challenging physicochemical property for the experimental laboratory12,13,14, even more so when working with oligomeric and/or highly labile integral membrane proteins15,16. The isolation of appropriate amounts of the membrane protein/enzyme with the highest possible quality for functional experimental assays and structural studies is highly desirable17. Membrane proteins, being inherently hydrophobic, are very difficult to purify, and the choice of detergent is usually one of the most important issues to be considered17,18,19,20. In this regard, the laboratory methods used to isolate membrane proteins typically cause some degree of damage to the 3D structural arrangement of the protein21. Some of these methods include the use of (a) sonication (high frequency/energy ultrasonic waves), (b) protein-solubilizing detergents (either harsh, medium, or gentle)22, (c) relatively high pressures in column chromatography and high-speed ultracentrifugation23, (d) precipitating molecules, (e) digestive enzymes, and others21. All these processes can contribute to or be the main cause of protein destabilization during purification21. In this respect, some protocols seem to work relatively well for a given membrane protein. However, optimization is always welcome when using new modern assays or methods that require a higher quality of membrane protein preparation to obtain satisfactory results24. Optimization steps may include, but are not limited to, improving construct design, finding optimal conditions for membrane protein expression, establishing better handling conditions (i.e., pH, temperature, etc.), finding the best compatible detergent, adjusting purification steps such as sonication time, centrifugation speed, reformulating buffer solutions by adding stabilizing agents, etc.25,26,27. Therefore, any change in the purification methodology that leads to an increase in the quality (purity) and activity of the purified membrane protein/enzyme is important.
In the P-type ATPase family, the yeast plasma membrane H+-ATPase appears to be one of the most labile members28,29. The H+-ATPase loses its ATP hydrolyzing activity upon dehydration (freeze-drying), heat shock, etc.28,29. The use of trehalose as a protein stabilizer was tested in the isolation of the plasma membrane H+-ATPase from the yeast K. lactis30,31; the H+-ATPase preparation obtained was of high purity and catalytically active. Importantly, this allowed the oligomeric state of the enzyme to be resolved by biochemical methods, revealing it to be a hexamer and later confirmed by cryo-electron microscopy31,32,33,34,35. Therefore, it seems likely that the delicate three-dimensional (3D) arrangement of membrane proteins can be lost under relatively harsh conditions during purification36. Biphasic inactivation kinetic is observed for H+-ATPase during heat-mediated inactivation29. In yeast cells, as in many other organisms, the disaccharide trehalose accumulates at high concentrations under environmental stress conditions37,38,39. Trehalose maintains membrane integrity and transport function by stabilizing proteins (both membrane-embedded and cytosolic) and cell membranes40,41. The stabilizing mechanism of trehalose has been extensively studied by several groups and by our laboratory42,43,44,45. Experiments with the H+-ATPase and other enzymes have shown that trehalose is the most effective protein stabilizer among mono- and disaccharides28,29. This led to its inclusion in the H+-ATPase purification protocol30. Recently, trehalose has also been used in the purification of the sarcoplasmic reticulum Ca2+-ATPase (SERCA) from rabbit fast-twitch muscle with good results in protein purity and activity46. Therefore, trehalose seems to be a good and appropriate additive for the purification of P-type ATPases and probably other membrane and cytosolic proteins.
For P-ATPases, the existence of structural cytoplasmic domains is an experimental advantage, especially for substrate/ligand interaction studies47; ATP binding has been studied in highly pure recombinant N-domains47,48,49, thus eliminating the technical considerations for purification of whole membrane enzymes47,48,50, among others51. Unfortunately, some functional (catalytic/energy conversion) and structural (subunit arrangement and interaction with other proteins) studies still require the whole P-ATPase52,53. In this regard, the purification of SERCA has been achieved by several research groups54,55,56,57,58. However, improvements can still be implemented46, for example, increasing the intactness of the purified ATPase59, avoiding protein denaturation/disruption of protein complexes25, increase solubilization without denaturation of the membrane protein (i.e., avoiding formation of macromolecular aggregates)60, lead to better compatibility with assays and other downstream analytical methods61 Furthermore, as new experimental strategies, additives, enzyme inhibitors, etc. appear in the scientific literature61,62,63,64,65,66, they sometimes need to be tested with the whole P-ATPase. This work describes the protocol for the purification of SERCA and the use of trehalose as an additive to stabilize the protein structure and the ATPase activity; i.e., in addition to increasing the enzyme (structural) quality, trehalose helps to prevent the loss of enzyme structure and activity during enzyme isolation and storage, which helps to save biological material and thus reduce the number of enzyme purifications.
Access restricted. Please log in or start a trial to view this content.
Protocol
All animal procedures were performed in accordance with international and local (NORMA Oficial Mexicana NOM-062-ZOO-1999) guidelines for the handling of animals in experimental laboratories51,67,68. Muscle tissue was obtained from wild-type Oryctolagus cuniculus from a local animal handling unit (INE/CITES/DGVS-ZOO-E0055-SLP-98)46. A veterinarian with expertise in laboratory animal management performed the initial muscle dissection and processing. The details of the reagents and the equipment used in this study are listed in the Table of Materials.
1. Isolation of sarcoplasmic reticulum from fast-twitch rabbit muscle (Timing, 7 - 9 h)
NOTE: For details on the procedure, refer to Champeil et al.69.
- Obtain fast-twitch muscle tissue (~70 g from both hind legs per animal) from wild-type Oryctolagus cuniculus.
NOTE: Prior visual identification of the muscles to be dissected is required70,71. - Macerate the fast-twitch muscle in a blender by suspending it in three volumes of 100 mM of KCl, with 1 min on and 1 min in ice-cold rest, repeat three times72.
- Remove the tissue debris by centrifugation (1500 × g) at 4 °C for 5 min; a second centrifugation (4200 × g) is performed if necessary.
- Collect the supernatant and homogenize it using a tissue grinder (10 strokes at low velocity, i.e., 500-1,500 rpm)72,73. Centrifuge the homogenate (10,000 × g) at 4 °C for 15 min.
NOTE: Care must be taken when homogenizing, as the use of a relatively high speed can result in damage to the system and the operator. - Centrifuge the supernatant (containing the sarcoplasmic reticulum) (33,000 × g) at 4 °C for 120 min.
- Suspend the pellets in 40 mL of 0.5 M of sucrose and centrifuge (12,000 × g) at 4 °C for 15 min. Dilute the supernatant (0.6 M of KCl and 0.15 M of sucrose) and centrifuge the suspension (34,000 × g) at 4 °C for 165 min.
- Suspend the pellet containing the sarcoplasmic reticulum vesicles (SRVs) sheets in 0.3 M of sucrose, 0.1 M of KCl, and 5 mM of Tris-HCl, pH 7.0. Determine the protein concentration by using the Lowry assay74 and human serum albumin as the protein standard.
- Aliquot the SRVs (30-32 mg/mL) in 1 mL volume. Store the SRVs samples at -72 °C for further processing.
NOTE: Protein yield is 100-110 mg SRVs per animal.
2. SERCA purification (Timing, 18 - 20 h)
NOTE: For details on the procedure, refer to Rivera-Morán et al.46.
- Dilute the SRVs suspension to 2 mg protein/mL using ice-cold 75 mM of Tris-HCl, pH 7.2, 0.6 M of KCl, 6 mM of EDTA, 1 mM of EGTA, and 0.1% (w/v) deoxycholate (DOC). Incubate the SRVs suspension on ice for 10 min and gentle agitation. Centrifuge the SRVs suspension (100,000 × g) at 4 °C for 60 min.
NOTE: DOC should be added drop by drop. - Suspend the pellets in <3 mL of 25 mM of Tris-HCl, pH 7.5, 0.3 M of KCl, 45% glycerol (v/v), and 2 mM EDTA. Homogenize the suspension and adjust to a final volume of 10 mL. Determine protein concentration and then, adjust it to 6.5 mg/mL. Add azolectin at 5 mg/mL and 0.85 (w/w) Zwittergent 3-14.
NOTE: Azolectin and Zwittergent 3-14 should be added drop by drop. - Homogenize the suspension and centrifuge (100,000 × g) at 4 °C for 60 min. Collect the supernatant and dilute 1:2 with 2 mM of EGTA (pH 7.2).
- Pour the suspension gently onto a discontinuous trehalose concentration gradient (45, 40, 35, and 30% w/v) in 10 mM of Tris-HCl, pH 7.0, 1 mM of EDTA, 0.1% deoxycholate and 1 mg/mL azolectin. Centrifuge (100,000 × g) at 4 °C for 14 h. Collect the transparent, slightly yellowish pellet formed at the bottom of the tubes.
NOTE: Both the formation of the trehalose gradient and the addition of the sample suspension must be performed using appropriate techniques. Overnight centrifugation is recommended. - Suspend gently the pellet in a small volume (<1.5 mL) of 25 mM of Tris-HCl, pH 7.5, 0.3 of M KCl, 45% glycerol, and 2 mM of EDTA. Determine protein concentration. Adjust the protein concentration by dilution to 2 mg/mL. Take aliquots (~50 µL) of the suspension and store the purified SERCA at −72 °C until its use.
- Perform SDS-PAGE of the purified SERCA72, and of protein samples taken at different steps of purification. Stain the gel with Coomassie Blue72.
NOTE: The use of 0.6 M trehalose instead of glycerol in the suspension buffer increases the stability of the enzyme during storage and freeze-thaw cycles28,29.
3. Assay for ATPase activity (Timing, 1 - 2 h)
- Enzyme-coupled assay: ATPase-Piruvate kinase-lactate dehydrogenase
- Prepare the reaction mixture (1 mL): 50 mM MOPS (pH 7.0), 1 mM EGTA, 80 mM KCl, 5 mM MgCl2, 3 mM CaCl2, 5 mM phosphoenolpyruvate, 250 µM NADH. Include variable ATP concentrations (0.001 to 0.25 mM).
- Homogenize carefully the reaction assay by vortexing. Incubate the reaction assay at 37 ◦C for 10 min. Add 0.9 U L-lactate dehydrogenase (LDH) and 1.5 U pyruvate kinase (PK).
- Initiate the ATPase reaction by the addition of 10 µg of SERCA.
- Determine the formation of NADH. Follow the change in absorbance intensity every second for 600 s at a wavelength (λ) of 340 nm over time using a spectrophotometer with a thermostatted cell holder.
NOTE: The time interval for the absorbance measurement can vary, but try to record as many data points as possible to draw the initial straight line of the ATPase reaction.
- Determine the rate of ATP hydrolysis
NOTE: For details on the procedure, refer to Rivera-Morán et al.46.- Calculate the slope value (Δabs/min) of the linear portion in each curve formed. Use the molar extinction coefficient (ε) of NADH (λ = 6,220 M−1·cm−1) to determine the ATPase activity (µmoles ATP hydrolyzed/min.mg prot.)
- Determine the kinetics parameters Km and Vmax
- Plot the velocity data versus ATP concentration using data analysis and graphing software.
- Fit the data by non-linear regression to the Michaelis-Menten Equation (Eq. 1)46:
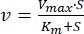
where v is the velocity, Vmax is the maximum velocity, [S] is the ATP concentration, and Km is the Michaelis-Menten constant. - Determine Km and Vmax by iteration.
NOTE: The inclusion of DOC may increase the ATPase rate response; testing different concentrations of DOC is recommended to find the optimum.
4. Labeling assay of SERCA with Fluorescein Isothiocyanate (FITC ) (Timing, 30 - 45 min, only for FITC labeling)
NOTE: For details on the procedure, refer to54,75,76.
- Suspend SERCA (20 µg) in 50 µL (final volume) of labeling buffer (100 mM of KCl, 5 mM MgCl2, and 30 mM of Tris-HCl, pH 8.9) containing 1 mM of FITC. Mix by vortexing.
- Incubate the samples at different times (15 min, 10 min, 7 min, 5 min, and 2 min) in the dark and at room temperature.
- Stop the labeling reaction by adding 1 volume of ice-cold stopping buffer (480 mM of sucrose and 48 mM of MOPS, pH 7.0), containing ATP (5 mM). Incubate on ice for 5 min in the dark.
- Subject the FITC-labeled SERCA to SDS-PAGE72. Expose the clear gels to UV light (λ = 302 nm). Photo document the result.
- Stain the gel with Coomassie blue. Photo document the result.
NOTE: Performing FITC labeling in a dark room may improve the quality of the fluorescence signal.
5. Circular dichroism (CD) spectra (Timing, 30 min)
NOTE: For details on the procedure, refer to11,77.
- Suspend SERCA (3 µM) in 400 µL of 10 mM phosphate buffer (pH 7.0) at 25 °C.
- Load the sample in a 0.1 cm path-length cell. Set far-UV spectra λ range between 190-260 nm. Set the internal resolution and the bandwidth to 1 nm.
- Record the CD signal at 50 nm/min at 25 °C.
Access restricted. Please log in or start a trial to view this content.
Results
SDS-PAGE of SERCA at different stages of purification (Figure 1). The Coomassie blue-stained gel shows the enrichment of the SERCA protein band (apparent molecular weight >100 kDa) as the purification protocol progresses. The protein band corresponding to SERCA shows a purity of >90% after centrifugation on a trehalose concentration gradient. Plot of ATP hydrolysis rate versus ATP concentration (Figure 2A). Michaelis-Menten ATPase kinetic (hyper...
Access restricted. Please log in or start a trial to view this content.
Discussion
Most molecules and ions cannot freely cross the cell membranes, e.g., proton (H+) requires a membrane transporter in the plasma membrane of a variety of organisms and organelles such as mitochondria83,84. Cell membranes are selective, and the molecules and ions that cross the cell membranes are diverse, so several types of membrane proteins can be found in the cell, such as (a) ABC transporters, (b) ion channels, (c) membrane-bound ATPases, (d) SLC tra...
Access restricted. Please log in or start a trial to view this content.
Disclosures
The author declares that he has no competing financial interests.
Acknowledgements
The author acknowledges the help of Edmundo Mata-Morales in editing the video, VM, Valentín de la Cruz-Torres in purifying the SRVs, Miguel A. Rivera-Moran in SERCA purification and analysis, and Juan C. Gonzalez-Castro, Franco E. Juarez, Alejandra Nevarez, Nicolas Rocha-Vizuet, and Jocelin I. Ramírez-Alonso in video production. No funds, grants, or other support were received to conduct this study.
Access restricted. Please log in or start a trial to view this content.
Materials
| Name | Company | Catalog Number | Comments |
| ATP | Sigma-Aldrich Corp | A2383 | |
| Azolectin from soybean | Sigma-Aldrich Corp | 44924 | |
| Benchtop UV transilluminator | Cole-Parmer | EW-97623-08 | Dual intensity High setting is ideal for analytical documentation. Low setting reduces photonicking or photobleaching of gel samples while doing preparative work. |
| CaCl2 • 2H2O | Sigma-Aldrich Corp | 223506 | |
| Coolpix B500 camera | Nikon Corp | S210 | |
| Coomassie brilliant blue G-250 | Bio-Rad | 1610406 | |
| Dodecyl maltoside | Sigma-Aldrich Corp | D4641 | |
| Ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid | Sigma-Aldrich Corp | E4378 | |
| Ethylenediaminetetraacetic acid tetrasodium salt dihydrate | Sigma-Aldrich Corp | E6511 | |
| Fluorescein isothiocyanate | Sigma-Aldrich Corp | F3651 | |
| KCl | JT Baker | 7447-40-7 | |
| MgCl2 • 6H2O | Sigma-Aldrich Corp | M9272 | |
| MOPS | Sigma-Aldrich Corp | M1254 | |
| NADH | Chem-Impex International Inc | 230 | |
| N-tetradecyl-N,N- dimethyl-3-ammonium-1-propanesulfonate | Sigma-Aldrich Corp | D0431 | |
| Phosphoenolpyruvate (PEP) | Chem-Impex International Inc | 9711 | |
| Rabbit muscle lactate dehydrogenase | Roche | 10003557103 | |
| Rabbit muscle pyruvate kinase | Sigma-Aldrich Corp | P1506 | |
| Sodium deoxycholate | Sigma-Aldrich Corp | D6750 | |
| Sodium dodecyl sulfate | Bio-Rad | 1610302 | |
| Spectropolarimeter | Jasco Corp. | Jasco J1500 | |
| Sucrose | Sigma-Aldrich Corp | 84100 | |
| Trehalose | Sigma-Aldrich Corp | T0167 | Dihydrate |
| Tris(hydroxymethyl)aminomethane hydrochloride | Sigma-Aldrich Corp | 857645 | |
| Ultracentrifuge | Beckman | Optima XPN | |
| UV/VIS spectrophotometer | Agilent Technologies | 8453 | The Agilent 8453 UV-Vis Spectrophotometer uses a photodiode array for simultaneous measurement of the complete ultra-violet to visible light spectrum |
| WiseStir HS- 30E | Daihan Scientific Co. | DH.WOS01010 | Ideal for all disperging and homogenizing applications, designed for tissue grinders. |
References
- Schuberth, C., Wedlich-Söldner, R. Building a patchwork - The yeast plasma membrane as model to study lateral domain formation. Biochim Biophys Acta Mol Cell Res. 1853 (4), 767-774 (2015).
- Douglas, L. M., Konopka, J. B. Fungal membrane organization: The eisosome concept. Annu Rev Microbiol. 68 (1), 377-393 (2014).
- Borrell, J. H., Domènech, Ò, Keough, K. M. W. Membrane protein - Lipid interactions: Physics and Chemistry in the Bilayer. , Springer International Publishing. (2016).
- Moraes, I., Evans, G., Sanchez-Weatherby, J., Newstead, S., Stewart, P. D. The next generation in membrane protein structure determination. , Springer International Publishing. (2016).
- White, S. H. Biophysical dissection of membrane proteins. Nature. 459 (7245), 344-346 (2009).
- Lacapère, J. -J. Membrane protein structure determination. Methods Mol Biol. , Humana Press. 459(1002).
- Palmgren, M. G. Plant plasma membrane H+-ATPases: Powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol. 52 (1), 817-845 (2001).
- Yatime, L., et al. P-type ATPases as drug targets: Tools for medicine and science. Biochim Biophys Acta Bioenerg. 1787 (4), 207-220 (2009).
- Drews, J. Tetrahydrobiopterin biosynthesis as an off-target of sulfa drugs. Science. 287 (5460), 1960-1964 (2000).
- Xu, Z., Meshcheryakov, V. A., Poce, G., Chng, S. -S. MmpL3 is the flippase for mycolic acids in mycobacteria. Proc Natl Acad Sci USA. 114 (30), 7993-7998 (2017).
- Siligardi, G., Hussain, R., Patching, S. G., Phillips-Jones, M. K. Ligand- and drug-binding studies of membrane proteins revealed through circular dichroism spectroscopy. Biochim Biophys Acta Biomembr. 1838 (1 PARTA), 34-42 (2014).
- Rawlings, A. E. Membrane proteins: Always an insoluble problem. Biochem Soc Trans. 44 (3), 790-795 (2016).
- Popot, J. -L. Membrane proteins in aqueous solutions. , Springer International Publishing. 708(2018).
- Palmgren, M. G., Sommarin, M., Ulvskov, P., Larsson, C. Effect of detergents on the H+-ATPase activity of inside-out and right-side-out plant plasma membrane vesicles. Biochim Biophys Acta Biomembr. 1021 (2), 133-140 (1990).
- McBride, Z., Chen, D., Reick, C., Xie, J., Szymanski, D. B. Global analysis of membrane-associated protein oligomerization using protein correlation profiling. Mol Cell Proteomics. 16 (11), 1972-1989 (2017).
- Yoneda, J. S., et al. Multimeric species in equilibrium in detergent-solubilized Na, K-ATPase. Int J Biol Macromol. 89, 238-245 (2016).
- Ohlendieck, K. Extraction of membrane proteins. Protein Purif Protocols. 59, 283-294 (2004).
- Champeil, P., et al. A robust method to screen detergents for membrane protein stabilization, revisited. Anal Biochem. 511, 31-35 (2016).
- Gimpl, K., Klement, J., Keller, S. Characterising protein/detergent complexes by triple-detection size-exclusion chromatography. Biol Proced Online. 18, 4(2016).
- le Maire, M., et al. Gel chromatography and analytical ultracentrifugation to determine the extent of detergent binding and aggregation, and Stokes radius of membrane proteins using sarcoplasmic reticulum Ca2+-ATPase as an example. Nat Protoc. 3 (11), 1782-1795 (2008).
- Du, M., et al. Progress, applications, challenges and prospects of protein purification technology. Front Bioeng Biotechnol. 10, 1-26 (2022).
- Slotboom, D. J., Duurkens, R. H., Olieman, K., Erkens, G. B. Static light scattering to characterize membrane proteins in detergent solution. Methods. 46 (2), 73-82 (2008).
- Harding, S. E. Analytical ultracentrifugation as a matrix-free probe for the study of kinase related cellular and bacterial membrane proteins and glycans. Molecules. 26, 6080(2021).
- Chen, Y. -C., et al. Thermal stability, storage and release of proteins with tailored fit in silica. Sci Rep. 7 (1), 46568(2017).
- Montigny, C. C., Arnou, B., Marchal, E., Champeil, P. Use of glycerol-containing media to study the intrinsic fluorescence properties of detergent-solubilized native or expressed SERCA1a. Biochemistry. 47 (46), 12159-12174 (2008).
- Simongini, M., Puglisi, A., Genovese, F., Hochkoeppler, A. Trehalose counteracts the dissociation of tetrameric rabbit lactate dehydrogenase induced by acidic pH conditions. Arch Biochem Biophys. 740, 109584(2023).
- Shivanna, B. D., Rowe, E. S. Preservation of the native structure and function of Ca2+-ATPase from sarcoplasmic reticulum: Solubilization and reconstitution by new short-chain phospholipid detergent 1,2-diheptanoyl-sn-phosphatidylcholine. Biochem J. 325 (2), 533-542 (1997).
- Sampedro, J. G., Guerra, G., Pardo, J. P., Uribe, S. Trehalose-mediated protection of the plasma membrane H+-ATPase from Kluyveromyces lactis during freeze-drying and rehydration. Cryobiology. 37 (2), 131-138 (1998).
- Sampedro, J. G., Cortés, P., Muñoz-Clares, R. A., Fernández, A., Uribe, S. Thermal inactivation of the plasma membrane H+-ATPase from Kluyveromyces lactis. Protection by Trehalose. Biochim Biophys Acta Proteins Proteomics. 1544 (1-2), 64-73 (2001).
- Sampedro, J. G., et al. Fluorescence quenching by nucleotides of the plasma membrane H+-ATPase from Kluyveromyces lactis. Biochemistry. 46 (18), 5616-5622 (2007).
- Ruiz-Granados, Y. G., De La Cruz-Torres, V., Sampedro, J. G. The oligomeric state of the plasma membrane H+-ATPase from Kluyveromyces lactis. Molecules. 24 (5), 958(2019).
- Zhao, P., Zhao, C., Chen, D., Yun, C., Li, H., Bai, L. Structure and activation mechanism of the hexameric plasma membrane H+-ATPase. Nat Commun. 12 (1), 6439(2021).
- Auer, M., Scarborough, G. A., Kühlbrandt, W. Surface crystallization of the plasma membrane H+-ATPase on a carbon support film for electron crystallography. J Mol Biol. 287 (5), 961-968 (1999).
- Hennessey, J. P., Scarborough, G. A. Secondary structure of the Neurospora CRASSA plasma membrane H+-ATPase as estimated by circular dichroism. J Biol Chem. 263 (7), 3123-3130 (1988).
- Scarborough, G. A. Crystallization, structure and dynamics of the proton-translocating P-type ATPase. J Exp Biol. 203 (Pt 1), 147-154 (2000).
- Sumida, K. H., et al. Improving protein expression, stability, and function with proteinMPNN. J Am Chem Soc. 146 (3), 2054-2061 (2024).
- Eleutherio, E., Panek, A., De Mesquita, J. F., Trevisol, E., Magalhães, R. Revisiting yeast trehalose metabolism. Curr Genet. 61 (3), 263-274 (2015).
- Attfield, P. V. Trehalose accumulates in Saccharomyces cerevisiae during exposure to agents that induce heat shock response. FEBS Lett. 225 (1-2), 259-263 (1987).
- Marunde, M. R., et al. Improved tolerance to salt and water stress in Drosophila melanogaster cells conferred by late embryogenesis abundant protein. J Insect Physiol. 59 (4), 377-386 (2013).
- Crowe, J. H. Anhydrobiosis: An unsolved problem with applications in human welfare. Subcell Biochem. 71, 263-280 (2015).
- Crowe, J. H., Crowe, L. M., Chapman, D. Preservation of membranes in anhydrobiotic organisms: The role of trehalose. Science. 223 (4637), 701-703 (1984).
- Sampedro, J. G., Rivera-Moran, M. A., Uribe-Carvajal, S. Kramers' theory and the dependence of enzyme dynamics on trehalose-mediated viscosity. Catalysts. 10 (6), 1-19 (2020).
- Sampedro, J. G., Uribe, S. Trehalose-enzyme interactions result in structure stabilization and activity inhibition. The role of viscosity. Mol Cell Biochem. 256 - 257 (1-2), 319-327 (2004).
- Crowe, J. H. Trehalose as a "chemical chaperone": Fact and fantasy. Adv Exp Med Biol. 594, 143-158 (2007).
- Crowe, J. H., Carpenter, J. F., Crowe, L. M., Anchordoguy, T. J. Are freezing and dehydration similar stress vectors? A comparison of modes of interaction of stabilizing solutes with biomolecules. Cryobiology. 27 (3), 219-231 (1990).
- Rivera-Morán, M. A., Sampedro, J. G. Isolation of the sarcoplasmic reticulum Ca2+-ATPase from rabbit fast-twitch muscle. Methods Protoc. 6 (5), 102(2023).
- Páez-Pérez, E. D., De La Cruz-Torres, V., Sampedro, J. G. Nucleotide binding in an engineered recombinant Ca2+-ATPase N-domain. Biochemistry. 55 (49), 6751-6765 (2016).
- Ramírez-Alonso, J. I., Sampedro, J. G. Effect of cations on ATP binding to the N-domain of Na+, K+-ATPase. J Fluoresc. , (2024).
- De la Cruz-Torres, V., Cataño, V., Olivo-Rodríguez, M., Sampedro, J. G. ANS Interacts with the Ca2+-ATPase nucleotide binding site. J Fluoresc. 30 (3), 483-496 (2020).
- Sampedro, J. G., Nájera, H., Uribe-Carvajal, S., Ruiz-Granados, Y. G. Mapping the ATP binding site in the plasma membrane H+-ATPase from Kluyveromyces lactis. J Fluoresc. 24 (6), 1849-1859 (2014).
- Kiani, A. K., et al. Ethical considerations regarding animal experimentation. J Prev Med Hyg. 63 (2 Suppl 3), E255-E266 (2022).
- Ruiz-Granados, Y., De La Cruz-Torres, V., Sampedro, J. The oligomeric state of the plasma membrane H+-ATPase from Kluyveromyces lactis. Molecules. 24 (5), 958(2019).
- Wang, S., et al. Structural basis for sarcolipin's regulation of muscle thermogenesis by the sarcoplasmic reticulum Ca2+-ATPase. Sci Adv. 7 (48), eabi7154(2021).
- Champeil, P., et al. ATP regulation of sarcoplasmic reticulum Ca2+-ATPase. Metal-free ATP and 8-bromo-ATP bind with high affinity to the catalytic site of phosphorylated ATPase and accelerate dephosphorylation. J Biol Chem. 263 (25), 12288-12294 (1988).
- Møller, J. V., Olesen, C. Preparation of Ca(2+)-ATPase1a enzyme from rabbit sarcoplasmic reticulum. Methods Mol Biol. 1377, 11-17 (2016).
- Wuytack, F., De Schutter, G., Casteels, R. Partial purification of (Ca2+ + Mg2+)-dependent ATPase from pig smooth muscle and reconstitution of an ATP-dependent Ca2+-transport system. Biochem J. 198 (2), 265-271 (1981).
- Mandal, A., et al. Solubilization, purification and reconstitution of Ca2+-ATPase from bovine pulmonary artery smooth muscle microsomes by different detergents: Preservation of native structure and function of the enzyme by DHPC. Biochim Biophys Acta Gen Subj. 1760 (1), 20-31 (2006).
- Inesi, G., Ma, H., Hua, S., Toyoshima, C. Characterization of Ca2+ ATPase residues involved in substrate and cation binding. Ann NY Acad Sci. 986 (1), 63-71 (2003).
- Van Winkle, W. B., Pitts, B. J., Entman, M. L. Rapid purification of canine cardiac sarcoplasmic reticulum Ca2+-ATPase. J Biol Chem. 253 (24), 8671-8673 (1978).
- Ruiz-Granados, Y. G., De La Cruz-Torres, V., Sampedro, J. G. The oligomeric state of the plasma membrane H+-ATPase from Kluyveromyces lactis. Molecules. 24 (5), 958(2019).
- Teramoto, N., Sachinvala, N. D., Shibata, M. Trehalose and trehalose-based polymers for environmentally benign, biocompatible, and bioactive materials. Molecules. 13 (8), 1773-1816 (2008).
- Dao, T. T., et al. Demethoxycurcumin is a potent inhibitor of P-type ATPases from diverse kingdoms of life. PLOS ONE. 11 (9), e0163260(2016).
- Bleeker, N. P., Cornea, R. L., Thomas, D. D., Xing, C. A novel SERCA inhibitor demonstrates synergy with classic SERCA inhibitors and targets multidrug-resistant AML. Mol Pharmaceutics. 10 (11), 4358-4366 (2013).
- Michelangeli, F., East, J. M. A diversity of SERCA Ca2+ pump inhibitors. Biochem Soc Trans. 39 (3), 789-797 (2011).
- Tadini-Buoninsegni, F., Smeazzetto, S., Gualdani, R., Moncelli, M. R. Drug interactions with the Ca2+-ATPase from Sarco(Endo)Plasmic Reticulum (SERCA). Front Mol Biosci. 5, 36(2018).
- Clausen, J. D., et al. Elucidation of antimicrobial activity and mechanism of action by N-substituted carbazole derivatives. Bioorg Med Chem Lett. 27 (19), 4564-4570 (2017).
- Leary, S., et al. AVMA guidelines for the euthanasia of animals: 2020 Edition, Members of the Panel on Euthanasia AVMA Staff Consultants. , AVMA. Schaumburg, IL. (2020).
- de Aluja, A. S. Laboratory animals and official Mexican norms (NOM-062-ZOO-1999). Gac Med Mex. 138 (3), 295-298 (2002).
- Champeil, P., Buschlen-Boucly, S., Bastide, F., Gary-Bobo, C. Sarcoplasmic reticulum ATPase. Spin labeling detection of ligand-induced changes in the relative reactivities of certain sulfhydryl groups. J Biol Chem. 253 (4), 1179-1186 (1978).
- Skalec, A., Janeczek, M., Czerski, A. Anatomy and histology of the rabbit common calcanean tendon. J Vet Med Series C: Anat Histol Embryol. 48 (5), 466-475 (2019).
- Janes, L. E., Mioton, L. M., Fracol, M. E., Ko, J. H. An in vivo comparison: Novel mesh suture versus traditional suture-based repair in a rabbit tendon model. J Hand Surg Glob Online. 4 (1), 32-39 (2022).
- Bollag, D. M., Rozycki, M. D., Edelstein, S. J. Protein Methods. , Wiley. (1996).
- Gagné, F. Tissue preparation and subcellular fractionation techniques. Biochem Ecotoxicol. , 21-31 (2014).
- Lowry, O. H., Rosebrough, N. J., Farr, A. L., Randall, R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 193 (1), 265-275 (1951).
- Autry, J. M., Rubin, J. E., Svensson, B., Li, J. L., Thomas, D. D. Nucleotide activation of the Ca-ATPase. J Biol Chem. 287 (46), 39070-39082 (2012).
- Winters, D. L., Autry, J. M., Svensson, B., Thomas, D. D. Interdomain fluorescence resonance energy transfer in SERCA probed by cyan-fluorescent protein fused to the actuator domain. Biochemistry. 47 (14), 4246-4256 (2008).
- Miles, A. J., Wallace, B. A. Circular dichroism spectroscopy of membrane proteins. Chem Soc Rev. 14 (45), 1130-1135 (2016).
- Scofano, H. M., Vieyra, A., de Meis, L. Substrate regulation of the sarcoplasmic reticulum ATPase. Transient kinetic studies. J Biol Chem. 254 (20), 10227-10231 (1979).
- Yamamoto, T., Tonomura, Y. Reaction mechanism of the Ca++-dependent ATPase of sarcoplasmic reticulum from skeletal muscle: I. Kinetic studies. J Biochem. 62 (5), 558-575 (1967).
- Murphy, A. J. Affinity labeling of the active site of the Ca2+-ATPase of sarcoplasmic reticulum. Biochim Biophys Acta - Biomembr. 946 (1), 57-65 (1988).
- Csermely, P., Katopis, C., Wallace, B. A., Martonosi, A. The E1→E2 transition of Ca2+-transporting ATPase in sarcoplasmic reticulum occurs without major changes in secondary structure. A circular-dichroism study. Biochem J. 241 (3), 663-669 (1987).
- Abràmoff, M. D., Magalhães, P. J., Ram, S. J. Image processing with ImageJ. Biophotonics Int. 11 (7), 36-42 (2004).
- Scarborough, G. A. The plasma membrane proton-translocating ATPase. Cell Mol Life Sci. 57 (6), 871-883 (2000).
- Llopis, J., McCaffery, J. M., Miyawaki, A., Farquhar, M. G., Tsien, R. Y. Measurement of cytosolic, mitochondrial, and Golgi pH in single living cells with green fluorescent proteins. Proc Natl Acad Sci. 95 (12), 6803-6808 (1998).
- Kanai, R., Vilsen, B., Cornelius, F., Toyoshima, C. Crystal structures of Na+, K+-ATPase reveal the mechanism that converts the K+-bound form to Na+-bound form and opens and closes the cytoplasmic gate. FEBS Lett. 597 (15), 1957-1976 (2023).
- Palmgren, M. P-type ATPases: Many more enigmas left to solve. J Biol Chem. 299 (11), 105352(2023).
- Frauenfelder, H. Ask not what physics can do for biology-Ask what biology can do for physics. Phys Biol. 11 (5), 053004(2014).
- Frauenfelder, H. The physics of proteins. Annu Rev Biophys Biomol Struct. 36, Springer. New York. New York, NY. (2010).
- Narhi, L. O. Biophysics for Therapeutic Protein Development. , Springer Science+Business Media. (2013).
- East, J. M. Purification of a membrane protein (Ca2+/Mg2+-ATPase) and its reconstitution into lipid vesicles. Methods Mol Biol. 27, 87-94 (1994).
- Lee, E. -H., et al. Enhancement of enzyme activity and stability by poly(γ-glutamic acid). Polymer J. 42 (10), 818-822 (2010).
- Norrild, R. K., et al. Increasing protein stability by inferring substitution effects from high-throughput experiments. Cell Rep Methods. 2 (11), 100333(2022).
- Song, Z., Zhang, Q., Wu, W., Pu, Z., Yu, H. Rational design of enzyme activity and enantioselectivity. Front Bioeng Biotechnol. 11, 1-14 (2023).
- Jidenko, M., Lenoir, G., Fuentes, J. M., le Maire, M., Jaxel, C. Expression in yeast and purification of a membrane protein, SERCA1a, using a biotinylated acceptor domain. Protein Expr Purif. 48 (1), 32-42 (2006).
- Hua, S., Ma, H., Lewis, D., Inesi, G., Toyoshima, C. Functional role of "N" (nucleotide) and "P" (phosphorylation) domain interactions in the sarcoplasmic reticulum (SERCA) ATPase. Biochemistry. 41 (7), 2264-2272 (2002).
- Pang, Y. -H., Chen, J. -W. Anisodamine causes the changes of structure and function in the transmembrane domain of the Ca2+-ATPase from sarcoplasmic reticulum. Biosci Biotechnol Biochem. 68 (1), 126-131 (2004).
Access restricted. Please log in or start a trial to view this content.
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved