A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Hellgrammite as a Non-Conventional Biomonitor for Surface Water and Environmental Health Assessment
In This Article
Summary
This protocol describes the use of biomarkers for the early detection of deleterious impacts in aquatic ecosystems. The biomarkers are closely related to sentinel traits, and their changes aid in detecting early-warning damages.
Abstract
The larvae of dobsonflies from the genus Corydalus, commonly known as Hellgrammites, are characterized by their notable size, extensive range of occurrence, and extended period of immaturity, which can last up to one year. Hellgrammites are clearly known to exhibit sensitivity to pollution and habitat structure impacts. Given these unique features, using Corydalus texanus larvae is highly suitable as reliable biomonitoring agents to assess the ecological integrity of aquatic ecosystems. This protocol aims to provide the necessary tools for C. texanus assessment and demonstrate their efficacy through a case study. Research findings have practical implications, indicating that C. texanus larvae exhibit early-warning responses to mining pollution, bioaccumulating high amounts of heavy metals such as Zn, Fe, and Al. The presence or absence of C. texanus populations may serve as a helpful indicator for identifying potential issues related to ecosystem health. The unconventional approach has shown early warnings of pollution in mining-impacted sites, highlighting the need for timely action to protect the environment. Given their unique traits, the use of C. texanus larvae is highly suggested as a reliable non-conventional bioindicator.
Introduction
Hellgramites are insect larvae from the order Megaloptera (Latreille, 1802), named dobsonflies or fishflies in their adult stage. A low diversity but widespread characterizes this group of top predator insect larvae from aquatic ecosystems1. Hellgramite species occur in well-defined biogeographic regions; therefore, it is relatively easy to identify species without a high taxonomic knowledge. Notably, the Corydalidae larvae possess the most prominent species from the Megaloptera order (20-90 mm body length)2, making hellgramite visible to the naked eye.
Hellgrammites play a crucial role in aquatic ecosystems as predators, with a powerful presence due to large chews denoting their impressive predatory shape. A dorsoventrally flattened body also joins with 7-8 pairs of filament gills along the body, and a head capsule with six stemmata per side makes hellgrammites fascinating organisms for entomologists and fans3. Adults of Corydalidae surprise and create an impression image to people due to their prominent size; however, they are entirely harmless. It is noteworthy that hellgrammites have the ability to persist in aquatic environments in their larval stage for a significant duration.
The phenotypical features of hellgrammites allow a particular chance to highlight their role in aquatic ecosystems; nevertheless, their indicator potential is the most wanted feature for aquatic ecologists. Vast knowledge of their bioindicator potential is highlighted in aquatic ecosystems because their occurrence relates to good health conditions in their habitats due to their intolerance to organic pollution in surface waters4,5,6,7,8.
Most Corydalidae megalopterans live in high-speed running waters as riffles and substrates predominated by cobble and pebbles, but hellgrammites also occur in low-gradient streams with snags and sand substrates, as well as in lentic habitats such as lakes3,9,10. Their wide range of occurrence mirrors the critical traits of a top predator and their ability to colonize several habitats aimed by their effective life history strategies11. Hellgramites linked their traits with the dynamics of aquatic ecosystems; thus, strategies such as adaptation to aerial respiration by spiracles (in addition to their ventral tufts of tracheal gills) are proper of the Corydalidae strategies10.
Hellgrammites inhabit particular ecosystems and exhibit rapid responses to deviations from established patterns, thereby serving as an early-warning system7. The reactions of these wildlife organisms can be employed as a valuable tool to assess the impact of pollution on aquatic ecosystems, particularly in the case of non-target pollutant mixtures. Some deleterious effects on living organisms have been recognized in ecosystems because of individual toxicity of chemicals, but the effect of pollutant mixtures shall be identified. The early-warning responses from hellgrammites may make it possible to identify deleterious effects by giving a reference to the impacts of a mixture of pollutants or even when individual effects of pollutants recognize No Observable Effect Concentration (NOEC)12.
Several model organisms have been used for experimental acute and chronic tests; however, they are cultured and maintained under controlled conditions13. Controlled conditions make them unable to identify the non-target effects of several pollutants they are exposed to. Also, NOEC is frequently recognized due to the complexity of the pollutant's mixture. For that reason, in the last decades, non-model native species with non-target effects were recognized to screen systems, which are essential to carrying out ecotoxicological research14. Consequently, hellgrammites seem able to assess the deleterious effects of pollution in aquatic ecosystems. Traits such as their biology, genetics, and physiology, among others, make non-model organisms suitable for impact assessment in ecosystems14.
This protocol aims to establish a novel biomonitoring tool using a non-model organism that can detect early-warning signals in response to non-target pollution mixtures. To achieve the best results, the traits exhibited by the larvae of the model species, C. texanus, have been comprehensively considered and integrated into the analysis15.
Protocol
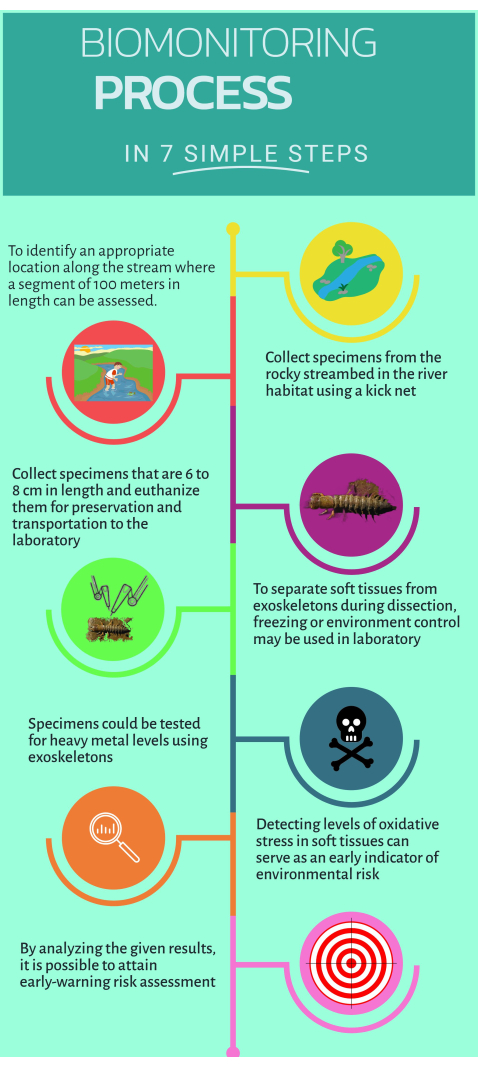
Figure 1: Quick step-by-step guide on how to use the non-conventional biomonitor Corydalus texanus for implementation in aquatic ecosystems. Please click here to view a larger version of this figure.
1. Fieldwork
- Identify study sites located upstream and downstream of the pollution source where suspected or evident impacts are recognized (i.e., punctual or diffuse emissions).
NOTE: The present protocol included 15 study sites in four main rivers: 1) Extoraz River (sites PB, EZ, RQ, BC); 2) Escanela-Jalpan River (ES, EN, AH, JL, PI, PA); 3) Ayutla River (AY); and 4) Santa María River (SM, AT). - Search and locate at least one reference site (a place under minor or absence of impacts) and define it as a study site.
NOTE: For the current study sites BC, ES, SM, AY and AT were considered as reference sites. - Recognize certain attributes of habitats that increase the likelihood of finding individuals of C. texanus. These include gravel substrate, rocky habitats with steeper gradients, high-speed flows, well-oxygenated waters, high canopy density, and habitats in highlands and lowlands.
- Enclose a 100 m section of the stream to identify appropriate habitats for biomonitoring16.
- Get inside the stream to collect samples.
- Sample at least six individuals of C. texanus in habitats such as riffles, pebbles, clear waters, and debris using a kick net with a mesh size of 500 µm.
- Remove rocks in the direction of the water flow and transfer the larvae into the net.
- Handle the kick net with sampled larvae and transfer them to designated bottles for temporary housing. Handle the net with caution and fold the edges towards the interior to prevent any loss of larvae.
- Measure the head width of sampled larvae using a caliper to identify instars IX to XI (within the range of 6 mm to 10 mm).
- Purge larvae by immersing them in a bottle containing stream water for 30 min.
- Place samples in plastic bottles with clear labeling and then euthanize them by immersion in liquid nitrogen (-75 °C) in a cryogenic tank.
- Transport and store the samples securely at -45 °C in the ultra-freezer at the laboratory.
- Using a polyethylene bottle, take a stream water sample of 200 mL, add 1 mL of nitric acid, and store the sample at 4 °C for subsequent heavy metal quantification in the laboratory.
2. Dissection of the sampled larvae and tissue separation
- Put the plastic dissection tray on ice and wait until it reaches 4 °C, using a thermometer to check.
- Take samples from the ultra-freezer and put them on the dissection tray to start dissecting.
- Select target tissues: muscle, branches, neural tube, and exoskeleton.
- Use a ceramic knife to remove the head and separate the tissues carefully. Open the body longitudinally and remove the gills on each side.
- Separate the exoskeletons using a smaller ceramic knife and clean the excess tissue. Place the exoskeletons in an oven and dry them at 80 °C for 48 h.
- After time is due, store dry exoskeletons for further analysis.
- Keep cleaning soft tissues under cold conditions for further analysis by microassays.
- Use a small ceramic knife to extract soft tissues from fat. Separate and pool each target tissue from individuals from the same sampling point.
- Store each tissue type in a 2 mL microtube, maintaining cold conditions throughout the process.
- To prepare each target tissue, weigh 100 mg of the tissue and add 1 mL of PBS solution with a pH of 7.4. Use a one-handed tissue tearor to homogenize each tissue. Typically, soft tissues require three cycles of 10 s each.
- Centrifuge sample at 1,000 x g for 5 min at 4 °C. Transfer the supernatant to a 1 mL microtube.
- Perform biomarker microassays.
3. Heavy metal quantification
- Weigh 250 mg of dried exoskeletons from at least six specimens in the specified developmental stage using an analytical balance.
- Grind exoskeletons in a glass mortar until they become finely powdered. Add 250 mL of ultrapure nitric acid to the powder reaction vessels.
- To digest the samples, transfer them to a digestion microwave at 180 °C for 30-45 min.
NOTE: Use the right microwave-safe container for the microwave brand to heat samples safely and effectively. Digestion time for tissue samples varies by microwave brand, taking 30-45 min. Some brands offer standardized methods. - Perform a microwave digestion method for water samples following the EPA3015A method, using 45 mL of the water sample and adding 5 mL of nitric acid.
- Measure heavy metals and metalloids in digested samples (water and tissues) using inductively coupled plasma optical emission spectroscopy (ICP-OES), including Hg, Cu, Pb, Zn, Fe, Cr, Cd, Mn, Ba, and As.
NOTE: Use argon grade 5 and standard solutions of each metal and metalloid to construct a calibration curve.
4. Microassays for the assessment of oxidative stress and biomarkers
- Preparing reagents
- Mix 6 g of NaH2PO4 with 1000 mL of ultrapure water to make a 50 mM Monobasic Phosphate Buffer. Store in a flask at 2-8 °C indefinitely.
- To make 8.1% SDS, dissolve 450 mg of sodium dodecyl sulfate (SDS) in 5 mL ultrapure water. Prepare just before use.
- Dilute 2 mL of trichloroethanoic acid (TCA) in 8 mL of ultrapure water (prepare fresh reagent when required).
- Dissolve 100 mg of tetrabutylammonium (TBA) in 10 mL of ultrapure water (prepare fresh reagent when required).
- To make 50 mM PBS pH 7.8, dissolve 97 mg of Na2HPO4 and 59 mg of NaH2PO4 in 10 mL of ultrapure water. Then, adjust the pH to 7.8 using NaOH to increase or HCl to decrease.
- Make a 200 µg/mL solution of bovine catalase by dissolving 8 mg of it in 40 µL of ultrapure water (prepare fresh reagent when required).
- To make a 50 mM TRIS/5 mM ethylenediaminetetraacetic acid (EDTA) solution, add 6.05 g of tris (hydroxymethyl)aminomethane (TRIS) and 0.073 g of EDTA to 45 mL of ultrapure water. Adjust pH to 7.6 with HCl and store at 4 °C until use.
- For 0.1 M glutathione reduced (GSH) (for 45 duplicated samples), weigh 0.0184 g of GSH and dissolve it in 600 µL of 10 mM HCl.
- To prepare 10 mM HCl, add 0.5 µL of concentrated HCl to 599.5 µL of ultrapure water.
- Add 28.5 µL of glutathione reductase to 1.4 mL of 0.1 M TRIS/HCl 0.5 mM EDTA pH 8.0.
- Mix 1.211 g of TRIS and 0.0146 g of EDTA with 90 mL of ultrapure water to prepare a 0.1 M TRIS/HCl and 0.5 mM EDTA solution at pH 8.0. Add HCl to adjust the pH if needed. The solution can be stored indefinitely at 2-8 °C.
- Weight 0.075 g of NADPH and add it to 2.25 mL of 1% NaHCO3 1 mM EDTA.
- Mix 1 g of NaHCO3 and 0.0282 g of EDTA in 100 mL of ultrapure water to prepare a 1% NaHCO3 and 1 mM EDTA solution. Store the solution at 4-8 °C for an undetermined time.
- Mix 2 µL of t-butyl hydroperoxide with 2 mL of ultrapure water to make a 7 mM solution. Mix immediately before use.
- Mix 1 g of bovine serum albumin (BSA) with 1 mL of ultrapure water to create a 1 µg/µL stock solution.
- Thiobarbituric acid reactive substances (TBARS) (lipid peroxidation, LPO)17
- Add 5 µL of tissue supernatant to a 1 mL microtube. Add 45 µL of 50 mM PBS (fresh and stock solution).
- Add 12.5 µL of 8.1% SDS (freshly prepared). Add 93.5 µL of 20% TCA pH 3.5 (freshly prepared).
- Add 93.5 µL of 1% TBA (freshly prepared). Complete the volume by adding 50.5 µL of ultrapure water. Close the tube's lid and vortex it for 30 s.
- Puncture the microtube lid with a needle and incubate in boiling water for 10 min.
- Cool the tube at room temperature (RT), and then centrifuge it at 1000 x g for 10 min.
- Add 150 µL of supernatant to a well in a 96-well microplate and measure absorbance at 530 nm using a microplate spectrophotometer.
- To quantify lipid peroxides, create an 8-point TBARS calibration curve (0-160 µM) using malondialdehyde bis (dimethyl acetal) as described in Table 1. Adjust the malondialdehyde (MDA) curve according to tissue type or suspected damage level.
- Express the results as nmol MDA eq·mg−1 protein.
- Superoxide dismutase antioxidant enzyme microassay (SOD)18
- Use a 96-well plate. Add 147 µL of 1 M TRIS/5 mM HCl EDTA pH 8 into two wells as blanks. Add 144 µL of 1 M TRIS/5 mM HCl EDTA pH 8 into other wells by duplicate.
- Take 3 µL from the supernatant and add it to the sample wells. Gently mix the contents on the plate several times.
- Add 3 µL of 10 mM pyrogallic acid to each well within 30 s. Mix gently immediately.
- Measure the absorbance at T0 (time = 0 s) and T10 (time = 10 s), at 420 nm and 25 °C.
- Determine SOD by calculating the percentage of pyrogallol inhibition in the sample using the following equation:
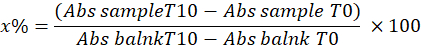
Where
x% = percentage of oxidation of pyrogallol by SOD in the sample; therefore, oxidation of pyrogallol is equal to 100 - x%
NOTE: Calculation of the SOD activity considering 1 IU is the volume that inhibits 50% of the pyrogallol oxidation as described below:
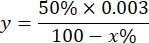
Where
y = sample volume on milliliter (inhibits 50% of pyrogallon)
50% = percentage of inhibition of pyrogallol equals 1U of SOD
0.003 = volume in milliliter of the sample
100 - x% = inhibition of pyrogallol in percentage by the sample - Finally, determine SOD activity with the equation:
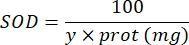
SOD = activity of SOD in international units per microgram of protein
Y = sample volume in that inhibits 50% of pyrogallol
100 = factor of conversion from deciliter to milliliter.
protein = protein concentration obtained from Bradford's quantification is expressed as micrograms per milliliter; it is necessary to divide by 10 to convert to grams per deciliter
- Catalase antioxidant enzyme microassay (CAT)19,20
- Use a 96-well microplate. Add 20 µL of sample supernatant into wells (by duplicate).
- Add 20 µL of fresh catalase (200 µg/mL) into wells as a standard (by duplicate). Add 100 µL of 50 mM PBS pH 7.8 to the sample and blank wells.
- Add 100 µL of 90 mM (30%) H2O2 into wells to start the reaction. After adding H2O2, read absorbance each 15 s for 2.5 min at 240 nm.
- Calculate activity using the equation:
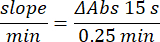
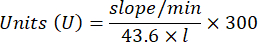
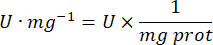
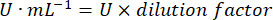
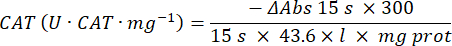
- Glutathione peroxidase enzyme microassay (GPx)21
- Use a 96-well microplate.
- Mix 240 µL of 0.1 M GSH solution, 1 200 µL of 10 U/mL GSH-Rd solution, and 1 200 µL of 4 mM NADPH. Keep the solution on ice.
- Add into wells 100 µL of 50 mM TRIS/HCl 5 mM EDTA pH 7.6. Add 1 µL of supernatant sample in duplicate. Tap the microplate and gently mix.
- Add 50 µL of the mix solution (step 4.5.2) and mix gently.
- Add 20 µL of the 7 mM t-butyl hydroperoxide solution into wells to start the reaction (it takes approximately 15 s to start the reaction in 16 duplicated samples). Quickly mix the microplate.
- Monitor absorbance at every 1 min interval for 5 min at 25 °C at 340 nm.
- Calculate GPx activity with the equation:
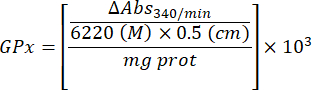
Where
GPx = activity in millimolar per minute per microgram of protein
ΔAbs340/min= Mean absorbance in delta
103 = molar to millimolar conversion
- mg prot = protein concentration from Bradford's determination expressed in microgram per microliter
- Protein quantification by Bradford's analysis22
- Perform a standard curve from bovine serum albumin (BSA) solutions, set from stock solution 1 µg/µL as shown in Table 2.
- Use a 96-well microplate. Add 190 µL of Bradford's reagent in the sample and standard wells.
- Add 10 µL of BSA in standard wells by duplicate. Add 10 µL of supernatant into the sample wells by duplicate.
- Gentle mix with the pipette tip.
- Read absorbance at 595 nm. Quantify the protein concentration with the standard curve.
Results
The results of the study reveal that heavy metals, specifically Aluminum (Al), Iron (Fe), and Zinc (Zn), have a detrimental impact on the environment, as evidenced by Figure 2. The detection of high levels of heavy metals in both tissue and water samples collected from all sites has led to an unfavorable outcome. Outliers were detected, with a Bioaccumulation Factor (BAF) of 600, notably in mining-intensive areas such as the Extoraz River (sites PB, EP, RQ, and BC shown in
Discussion
Although the use of C. texanus is optimal for evaluation, it is necessary to consider several aspects of its use and collection. Chosen study sites prove challenging, owing to several factors such as unfavorable weather conditions, geographical inaccessibility, high aridity levels, or insufficient security protocols in selected regions. Stated constraints and limitations can often present challenges when conducting fieldwork. When evaluating specific regions, particularly those in tropical streams, obtaining sam...
Disclosures
The authors disclosed no conflicts of interest present in their work.
Acknowledgements
The authors would like to express their sincere gratitude to CONAHCyT for providing the FONINS P 1931 grant, which greatly facilitated their research efforts. They would also thank the Secretaría de Investigación y Posgrado at Instituto Politécnico Nacional for the invaluable support provided through the SIP project grant (20200577). In addition, the first author wishes to acknowledge the generous post-graduate scholarship awarded by CONAHCyT, which enabled the team to conduct field trips and gather essential data. Finally, the authors would like to express their appreciation for the invaluable assistance of María Teresa García Camacho in the laboratory, without whom this project would not have been possible.
Materials
| Name | Company | Catalog Number | Comments |
| Analytical balance of 220 g | Ohaus | PR224/E | This balance is useful for weighing the extracted tissue from specimens. |
| Chest waders | LaCrosse | 700152M | We recommend using waders for sample collection. Alternatively, you can also use boots with rubber hip boots for sampling. |
| Cutting board | True | TRUE915121 | It is recommended to use white and plastic boards. |
| Forceps | DR Instruments | 112 | It is recommended to use a 12 pul forceps made of stainless steel. |
| Inductively Coupled Plasma-Optical Emission Spectroscopy System | Perkin Elmer | 7300 DV | A device for quantifying heavy metals using spectroscopy. |
| Kick net | LaMotte | 0021-P | An alternative method for making a kick net involves manually crafting one using a mesh with a thickness of 500 micrometers. |
| Liquid Nitrogen | NA | NA | NA |
| Liquid Nitrogen Dewar Static Cryogenic Container | BestEquip | DF0504 | It is recommended to use an aluminum tank with canisters. |
| Mortar and Pestle Set | Cole-Parmer | EW-63100-54 | This is a porcelain mortar and pestle used for grinding dry tissues. |
| Multiwave GO Plus | Anton Paar | C93IP001EN-E | Multiwave is a useful tool for digesting multiple samples. |
| Oven for drying | Fisher scientific | 506G | An incubator oven, also known as a dry tissue oven, is essential for drying tissues at temperatures of at least 80°C. |
| Precision scalpel | Xcelite by Weller | 037103-48768 | It is recommended to use a scalpel made of aluminum. |
| Tissue Homogenizer (tearer) | Kopro | K110000 | It is recommended to use a tissue tearer with a base. Some companies offer ultrasonic tearers, which may be the optimal choice. |
| Ultra-Low Temperature Chest Freezer | REVCO | CA89200-384 | Many companies provide freezers, but it is recommended to choose one with a storage temperature of at least -40°C. |
| Wide mouth plastic bottles | United Scientific | 81900 | Using polypropylene bottles with wide caps and mouths is strongly recommended. |
References
- Rivera-Gasperín, S. L., Ardila-Camacho, A., Contreras-Ramos, A. Bionomics and ecological services of Megaloptera larvae (Dobsonflies, Fishflies, Alderflies). Insects. 10 (4), 86 (2019).
- Cover, M. R., Seo, J. H., Resh, V. H. Life history, burrowing behavior, and distribution of Neohermes filicornis (Megaloptera: Corydalidae), a long-lived aquatic insect in intermittent streams. Western North Ame. Nat. 75 (4), 474 (2016).
- Martins, C. C., Ardila-Camacho, A., Rivera-Gasperín, S. L., Oswald, J. D., Liu, X., Contreras-Ramos, A. A world checklist of extant and extinct species of Megaloptera (Insecta: Neuropterida). Euro J of Tax. 812 (1), 1-93 (2022).
- Knight, A. W., Siegfried, C. A. The distribution of Corydalus Cornutus (Linnaeus) and Nigronia Serricornis (Say) (Megaloptera: Corydalidae) in Michigan. The Great Lakes Entomol. 10 (2), 1 (2017).
- . Water quality of Quebrada Cuecha: effects of the effluent from the Monteverde cheese factory Available from: https://digitalcommons.usf.edu/cgi/viewcontent.cgi?article=1662&context=tropical_ecology (2018)
- Cao, C. -. Q., Liu, Z., Chen, S. -. Z., Tong, C. The swimming behavior of the aquatic larva of Neoneuromus ignobilis (Megaloptera: Corydalidae: Corydalinae). Acta Entomol Sin. 55 (1), 133-138 (2012).
- Rico-Sánchez, A. E., Rodríguez-Romero, A. J., Sedeño-Díaz, J. E., López-López, E. Assessment of seasonal and spatial variations of biochemical markers in Corydalus sp. (Megaloptera: Corydalidae), a non-conventional biomonitor, in a mountain cloud forest in Mexico. Environ Sci Pollut Res Int. 27 (24), 30755-30766 (2020).
- Galindo-Pérez, E. J., et al. Cave macroinvertebrates as bioindicators of water quality. Water Sci Technol. 8 (5), 5-17 (2017).
- Dolin, P. S. The life history and ecology of Chauliodes Rastricornis Rambur and C. Pectinicornis. (Linnaeus)(Megaloptera: Corydalidae) in Greenbottom swamp, Cabell County, West Virginia. Marshall University. , (1980).
- Cover, M. R., Resh, V. H. Global diversity of dobsonflies, fishflies, and alderflies (Megaloptera; Insecta) and spongillaflies, nevrorthids, and osmylids (Neuroptera; Insecta) in freshwater. Hydrobiologia. 595 (1), 409-417 (2008).
- Barba-Álvarez, R., De la Lanza-Espino, G., Contreras-Ramos, A., González-Mora, I. Aquatic insects indicators of water quality in Mexico: case studies, Copalita, Zimatán and Coyula rivers, Oaxaca. Mexican Biodiversity Magazine. 84 (1), 381-383 (2013).
- Beyer, J., et al. Environmental risk assessment of combined effects in aquatic ecotoxicology: A discussion paper. Mar Env Res. 96, 81-91 (2014).
- Tišler, T., Zagorc-Končan, J. Comparative assessment of toxicity of phenol, formaldehyde, and industrial wastewater to aquatic organisms. Water Air Soil Pollut. 97, 315-322 (1997).
- Larramendy, M. Ecotoxicology and Genotoxicology: Non-traditional Aquatic Models. Royal Society of Chemistry. , (2017).
- Contreras-Ramos, A., Rosas, M. V. Biodiversity of Megaloptera and Raphidioptera in Mexico. Biodiversity Supplement of Mexico. 85, 257-263 (2014).
- Villamarín, C., Rieradevall, M., Paul, M. J., Barbour, M. T., Prat, N. A tool to assess the ecological condition of tropical high Andean streams in Ecuador and Peru: The IMEERA index. Ecol Indic. 29, 79-92 (2013).
- Uchiyama, M., Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 86 (1), 271-278 (1978).
- Marklund, S., Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 47 (3), 469-474 (1974).
- Aebi, H. Catalase. Methods Enzymol. 105, 121-126 (1984).
- Li, Y., Schellhorn, H. E. Rapid kinetic microassay for catalase activity. J Biomol Tech. 18 (4), 185-187 (2007).
- Kumar, P., Maurya, P. K. L-Cysteine efflux in erythrocytes as a function of human age: Correlation with reduced glutathione and total anti-oxidant potential. Rejuvenation Res. 16 (3), 179-184 (2013).
- Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72 (1-2), 248-254 (1976).
- Barbour, M. T., Stribling, J. B., Verdonschot, P. F. M. The multihabitat approach of USEPA's rapid bioassessment protocols: benthic macroinvertebrates. Limnetica. 25 (3), 839-850 (2006).
- Allan, J. D. . Stream Ecology: Structure and Function of Running Waters. , (2007).
- Barbour, M. T., Gerritsen, J., Snyder, B. D., Stribling, J. B. Rapid Bioassessment Protocols for Use in Streams and Wadeable Rivers: Periphyton, Benthic Macroinvertebrates and Fish. United States Environmental Protection Agency. , (1999).
- Wright, J. F., Armitage, P. D., Furse, M. T., Moss, D. The classification of sites on British rivers using macroinvertebrates. SIL Proceed, 1922-2010. 22 (3), 1939-1943 (1984).
- Rico-Sánchez, A. E., Rodríguez-Romero, A. J., Sedeño-Díaz, J. E., López-López, E., Sundermann, A. Aquatic macroinvertebrate assemblages in rivers influenced by mining activities. Sci Rep. 12 (1), 3209 (2022).
- Ramírez, A., Pringle, C. M., Wantzen, K. M. . Tropical Stream Conservation. Tropical Stream Ecology. , (2008).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved