Bacterial Growth Curve Analysis and its Environmental Applications
Overview
Source: Laboratories of Dr. Ian Pepper and Dr. Charles Gerba - The University of Arizona
Demonstrating Author: Luisa Ikner
Bacteria are among the most abundant life forms on Earth. They are found in every ecosystem and are vital for everyday life. For example, bacteria affect what people eat, drink, and breathe, and there are actually more bacterial cells within a person’s body than mammalian cells. Because of the importance of bacteria, it is preferable to study particular species of bacteria in the laboratory. To do this, bacteria are grown under controlled conditions in pure culture, meaning that only one type of bacterium is under consideration. Bacteria grow quickly in pure culture, and cell numbers increase dramatically in a short period of time. By measuring the rate of cell population increase over time, a “growth curve” to be developed. This is important when aiming to utilize or inoculate known numbers of the bacterial isolate, for example to enhance plant growth, increase biodegradation of toxic organics, or produce antibiotics or other natural products at an industrial scale.
Procedure
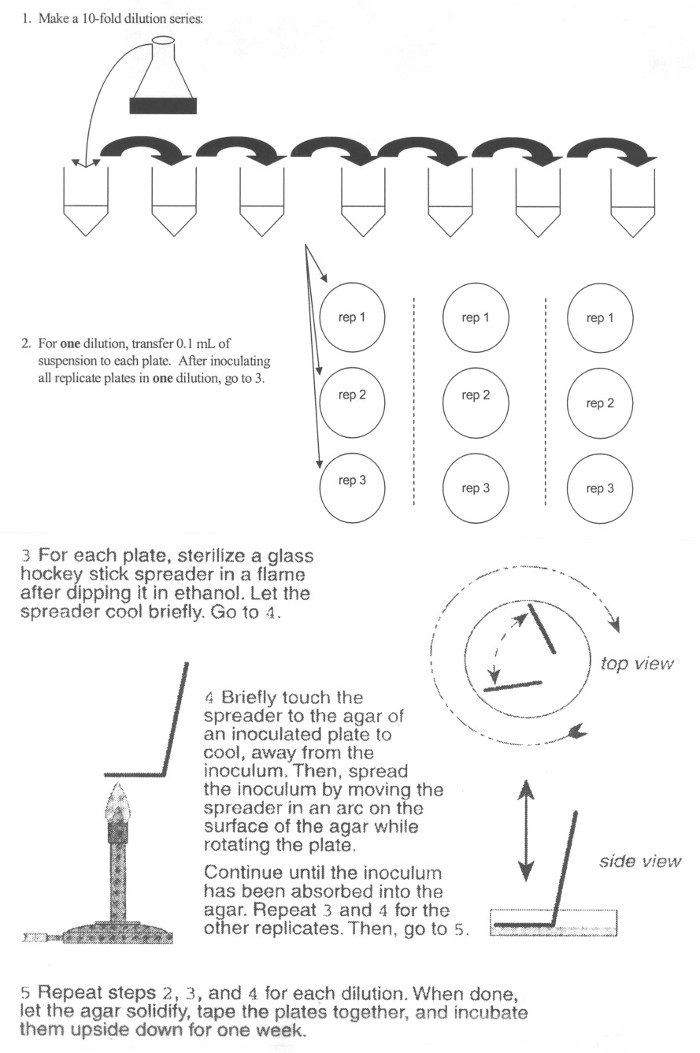
1. Collection of Bacterial Culture Aliquots
- One day before collection of growth time points, inoculate 20 mL of trypticase soy broth (TSB) medium in a 50-mL flask with E. coli.
- Incubate overnight at 27 °C. This relatively long incubation period results in a stationary phase population of wild type E. coli of approximately 109 CFU/mL.
- On the following day, use 100 µL of the prepared culture to inoculate 250 mL of TSB (in a 500-mL flask). Mix thoroughly
Results
Following a serial dilution plating experiment, the following data was obtained. At the beginning of exponential growth designated here as time t = 0, the initial concentration of bacterial cells is 1,000 CFU/mL. At time t = 6 h, the concentration of cells is 16,000 CFU/mL.
Now, X = 2n x X0
Where: X0 = initial concentration of cells = 1,000 CFU/mL
X = concentration of cells after time t = 16,000 CFU/mL
n
Application and Summary
Knowledge of bacterial growth kinetics and bacterial numbers in a culture medium is important from both a research and commercial point of view. In research, it is often critical to know the number of bacteria in a sample, so the experiment can be replicated, if need be, with the exact same numbers. For example, during experiments in which bacterial inoculants are added to a plot of soil, a minimum of 104 CFU needs to be added per gram of soil to get the desired effect, such as enhanced biodegradation of toxic
Tags
Skip to...
Videos from this collection:

Now Playing
Bacterial Growth Curve Analysis and its Environmental Applications
Environmental Microbiology
296.3K Views

Determination of Moisture Content in Soil
Environmental Microbiology
359.8K Views

Aseptic Technique in Environmental Science
Environmental Microbiology
126.5K Views

Gram Staining of Bacteria from Environmental Sources
Environmental Microbiology
100.5K Views

Visualizing Soil Microorganisms via the Contact Slide Assay and Microscopy
Environmental Microbiology
42.4K Views

Filamentous Fungi
Environmental Microbiology
57.6K Views

Community DNA Extraction from Bacterial Colonies
Environmental Microbiology
28.9K Views

Detecting Environmental Microorganisms with the Polymerase Chain Reaction and Gel Electrophoresis
Environmental Microbiology
44.6K Views

RNA Analysis of Environmental Samples Using RT-PCR
Environmental Microbiology
40.4K Views

Quantifying Environmental Microorganisms and Viruses Using qPCR
Environmental Microbiology
47.9K Views

Water Quality Analysis via Indicator Organisms
Environmental Microbiology
29.6K Views

Isolation of Fecal Bacteria from Water Samples by Filtration
Environmental Microbiology
39.4K Views

Detection of Bacteriophages in Environmental Samples
Environmental Microbiology
40.8K Views

Culturing and Enumerating Bacteria from Soil Samples
Environmental Microbiology
184.8K Views

Algae Enumeration via Culturable Methodology
Environmental Microbiology
13.8K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved