Column Chromatography
Overview
Source: Laboratory of Dr. Jimmy Franco - Merrimack College
Column chromatography is one of the most useful techniques for purifying compounds. This technique utilizes a stationary phase, which is packed in a column, and a mobile phase that passes through the column. This technique exploits the differences in polarity between compounds, allowing the molecules to be facilely separated.1 The two most common stationary phases for column chromatography are silica gel (SiO2) and alumina (Al2O3), with the most commonly used mobile phases being organic solvents.2 The solvent(s) chosen for the mobile phase are dependent on the polarity of the molecules being purified. Typically more polar compounds require more polar solvents in order to facilitate the passage of the molecules through the stationary phase. Once the purification process has been completed the solvent can be removed from the collected fractions using a rotary evaporator to yield the isolated material.
Principles
The sample mixture is placed on the top of the column and absorbed onto the top of the stationary phase. Subsequently, the mobile phase is applied to the column and used to elute the mixture through the stationary phase. Column chromatography exploits a molecule's polarity to separate the compounds. The difference in polarity leads to variances in the rate at which the molecules travel through the column, which effectively separates the compounds from one another. The mobile phase is collected in small fractions in test tubes as it elutes off the column, thus allowing for the isolation and purification of the compounds. Lastly, the solvent is removed using a rotary evaporator to yield the isolated compound(s).
Column chromatography's versatility and convenience has made it one of the most widely used techniques for purifying compounds. Unlike recrystallization (another commonly used purification technique) compounds purified with column chromatography do not have to be solid. Column chromatography is also capable of isolating a number of compounds from a mixture. Another advantage of column chromatograph is that very little needs to be known about the compound's physical properties in order to use this purification method, making this technique very valuable when synthesizing or isolating novel compounds, in which little is known about the compound(s).
Solvent
The rate at which a compound traverses through the column is highly dependent on the mobile phase being utilized. Typically, the more polar the solvent the faster the compounds will pass through the column. Polar solvents have a greater affinity for the solid phase, limiting the interactions between the compound(s) and the solid phase, allowing the compounds to elute more rapidly. Caution must be taken to ensure that the solvent system chosen for the column chromatography has the appropriate polarity to create separation between the compounds in the mixture. The solvent choice is crucial to successful separation using column chromatography. To identify an optimal solvent system, a series of thin layer chromatography (TLC) experiments should be conducted prior to performing the column chromatography experiment. In some case it may be necessary to use a binary solvent system.
Selecting a Solvent System
- Identify a solvent system that produces a retardation factor (Rf ) between 0.2–0.3 for the desired compound on a TLC plate.
- Start with either ethyl acetate or dichloromethane as the mobile phase for the TLC experiment.
- If the Rf is greater than 0.3, try less polar solvent, such as hexane. If the Rf is less than 0.2, try adding a small amount of a polar solvent such as methanol.
- The optimal solvent system may require a mixture of two solvents.
- Be careful not use more than 10% methanol as the mobile phase for a silica column.
Procedure
1. Silica Gel Slurry
- Pour the silica gel into an Erlenmeyer flask. The weight of the packing material should be roughly 50x that of the sample being separated. If the compounds being separated have very similar Rf values, then it may require using a larger amount of silica per sample, which is the case in this example.
- Place 10 g of silica in the Erlenmeyer flask, since 50 mg of sample (45 mg of fluorenone and 5 mg of tetraphenylporphyrin) are being isolated.
- Add the solvent system (hexane/dichloromethane, 70%: 30%) to the Erlenmeyer flask containing the silica gel. Add enough solvent to ensure that all of the silica gel is well solvated. The silica will not dissolve, but the mixture will be visually noticeable when solvated. Once the solvent has been added swirl the Erlenmeyer flask to ensure that all of the silica is well solvated.
2. Preparation of the Column
- Select the appropriately sized column. Typically the column should be filled about half way with silica gel slurry. The larger the sample being purified, the larger the column required.
- Plug the bottom of the column with a piece of glass wool. Using a long rod, make sure the wool is firmly lodged in the bottom of the column just above the stopcock.
- Once the wool is firmly in place, apply a thin layer of sand over the glass wool.
Note: If the column is equipped with a glass frit above the stopcock, this step should be omitted. - Clamp the column in the vertical position to a ring stand.
- Using a funnel, gently pour the prepared slurry of silica gel into the column. You may need to add additional solvent to transfer the slurry from the Erlenmeyer flask to the column. Using a pipette, wash down any silica gel that sticks to the sides of the column.
- As the silica gel is settling in the column, gently tap the sides of the column to ensure that the silica gel packs tightly and excludes any air bubbles.
- Open the stopcock and allow solvent to drain into a clean Erlenmeyer flask until just before the silica gel and the solvent front meet. The silica gel should never go dry until the procedure is complete.
- Place a thin layer of sand on top of the silica gel (Figure 1). Using a pipette, wash down any sand that may have stuck to the sides of the column.
- Drain any additional solvent until the sand is dry, but not down to the silica gel layer.
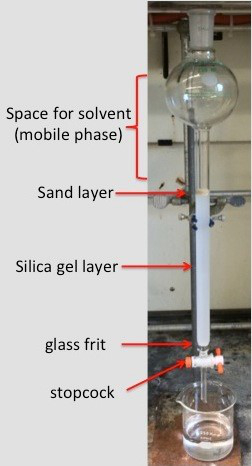
Figure 1. The proper setup for a column chromatography experiment prior to the addition of the sample.
3. Adding the Sample to the Column
- Dissolve the sample in the smallest amount of solvent possible (using the same solvent that was used to make the silica gel slurry).
- Using a pipette, gently add the sample to the top of the column.
- Once the sample has been applied to the top of the column, open the stopcock and allow the solvent to drain through the sand layer but not the silica gel layer. Use a very small amount of solvent to wash down any sample that may have clung to the sides of the column. Drain this additional solvent through the sand layer as well.
4. Eluting the Sample through the Column
- Using a pipette, very gently add 4–5 mL of solvent in such a manner that does not disturb the sand layer.
- Place a funnel at the top of the column and very slowly and gently fill the remainder of the column with solvent.
- Open the stopcock and allow the solvent to drain through the column.
- Begin collecting the mobile phase as it drains from the column into test tubes.
- Test tubes should be placed in a test tube rack in a sequential manner.
- Add additional solvent to the top of the column as needed until all the desired compounds have eluted from the column.
5. Recovering the Constituents
- If the compounds are colored, then they can be visually identified. However, if the compounds are colorless, they will have to be identified using ulta-visible (UV) light (if the compounds contain conjugation) or with the appropriate stain. The purity of the compounds can be verified using thin layer chromatography.
- Identify the test tubes that contain the desired compound(s).
- Merge all of the fractions that contain the desired isolated compound(s) into a pre-weighed round-bottom (RB) flask. Do this for each compound being isolated.
- Evaporate the solvent by placing the RB flask on the rotary evaporator.
- Once all of the solvent has been removed, weigh the RB with the dried product and subtract the initial weight of the RB to obtain a yield.
Results
The sample containing a mixture of tetraphenylporphyrin (TPP, 5 mg) and fluorenone (45 mg) has been successfully separated and each compound has been isolated. The TPP eluted first off the column as a dark purple-reddish band and the fluorenone subsequently eluted off the column as a yellow band (Figure 2). The eluted fractions were collected in test tubes and identified by their distinctive colors (Figure 3). The fractions containing the isolated compounds were merged into separate RBs and the solvent was removed using a rotary evaporator to afford highly pure TPP and fluorenone. The purity of the chromatographed compounds was validated by nuclear magnetic resonance (NMR) spectroscopy. Compounds can additionally be verified by melting point, but only if the melting point for the desired compound(s) has been previously determined.

Figure 2. As the compounds traverse through the stationary phase they begin to separate. In this experiment the TPP (dark purple-reddish band) travels through the column slightly faster than the fluorenone (yellow band).

Figure 3. As the compounds elute off the column they are collected in test tubes. The compounds being separated in this experiment are colored, so they can be visually identified.
Application and Summary
Summary
Column chromatography is a convenient and versatile method for purifying compounds. This method separates compounds based on polarity. By exploiting differences in the polarity of molecules, column chromatography can facilely separate compounds by the rate at which the compounds traverse through the stationary phase of the column. One of the benefits of column chromatography (especially when compared to recrystallization) is that very little about the compounds needs be known prior to the purification process. The other advantage to using column chromatography is that it can be used to purify both solids and oils, while recrystallization can only be used to purify solids. This technique can also be used to isolate a number of compounds from a mixture.
Applications
Column chromatography is one of the most convenient and widely used methods for purifying compounds. Often, synthetic reactions will produce multiple products and column chromatography can be used to isolate each of the compounds for further examination. Column chromatography is extremely valuable when synthesizing or isolating novel compounds, as very little needs to be known about a compound and its' physical properties prior to the purification process.
The pharmaceutical industry routinely uses column chromatography to purify compounds as part of its early stage drug development process.3 Often in these preliminary stages researchers will construct libraries of compounds around a lead compound, then subsequently use column chromatography to purify the newly synthesized compounds.4 The extensive use and versatility of this purification technique has prompted educators to incorporate the technique into the undergraduate curriculum.5,6
References
- Mayo, D. W.; Pike, R. M.; Forbes, D. C., Microscale organic laboratory : with multistep and multiscale syntheses. 5th ed.; J. Wiley & Sons: Hoboken, NJ; p xxi, 681 p (2011).
- Armarego, W. L. F.; Chai, C. L. L., Purification of laboratory chemicals. 5th ed.; Butterworth-Heinemann: Amsterdam; Boston; p xv, 609 p (2003).
- Silverman, R. B.; Holladay, M. W., The organic chemistry of drug design and drug action. Third edition / ed.; Elsevier/AP, Academic Press, is an imprint of Elsevier: Amsterdam ; Boston; p xviii, 517 pages (2014).
- Mortensen, D. S.; Perrin-Ninkovic, S. M.; Shevlin, G.; Elsner, J.; Zhao, J.; Whitefield, B. et. al. Optimization of a Series of Triazole Containing Mammalian Target of Rapamycin (mTOR) Kinase Inhibitors and the Discovery of CC-115. Journal of Medicinal Chemistry (2015).
- Davies, D. R.; Johnson, T. M., Isolation of Three Components from Spearmint Oil: An Exercise in Column and Thin-Layer Chromatography. Journal of Chemical Education,84 (2), 318 (2007).
- Taber, D. F.; Hoerrner, R. S., Column chromatography: Isolation of caffeine. Journal of Chemical Education, 68 (1), 73 (1991).
Tags
Skip to...
Videos from this collection:

Now Playing
Column Chromatography
Organic Chemistry
360.0K Views

Introduction to Catalysis
Organic Chemistry
34.5K Views

Assembly of a Reflux System for Heated Chemical Reactions
Organic Chemistry
167.3K Views

Conducting Reactions Below Room Temperature
Organic Chemistry
70.5K Views

Schlenk Lines Transfer of Solvents
Organic Chemistry
41.6K Views

Degassing Liquids with Freeze-Pump-Thaw Cycling
Organic Chemistry
56.1K Views

Preparing Anhydrous Reagents and Equipment
Organic Chemistry
79.3K Views

Purifying Compounds by Recrystallization
Organic Chemistry
708.4K Views

Separation of Mixtures via Precipitation
Organic Chemistry
157.7K Views

Solid-Liquid Extraction
Organic Chemistry
237.8K Views

Rotary Evaporation to Remove Solvent
Organic Chemistry
212.8K Views

Fractional Distillation
Organic Chemistry
334.3K Views

Growing Crystals for X-ray Diffraction Analysis
Organic Chemistry
32.4K Views

Performing 1D Thin Layer Chromatography
Organic Chemistry
289.7K Views

Nuclear Magnetic Resonance (NMR) Spectroscopy
Organic Chemistry
247.7K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved