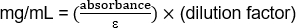Antibody Generation: Producing Monoclonal Antibodies Using Hybridomas
Overview
Source: Frances V. Sjaastad1,2, Whitney Swanson2,3, and Thomas S. Griffith1,2,3,4
1 Microbiology, Immunology, and Cancer Biology Graduate Program, University of Minnesota, Minneapolis, MN 55455
2 Center for Immunology, University of Minnesota, Minneapolis, MN 55455
3 Department of Urology, University of Minnesota, Minneapolis, MN 55455
4 Masonic Cancer Center, University of Minnesota, Minneapolis, MN 55455
Polyclonal antibodies are defined as a collection of antibodies directed against different antigenic determinants of an antigen or several antigens (1). While polyclonal antibodies are powerful tools for identifying biological molecules, there is one important limitation - they are unable to distinguish between antigens that share antigenic determinants. For example, when bovine serum albumin is used to immunize an animal, B cells with different surface Ig will respond to different antigenic determinants on bovine serum albumin. The result is a mixture of antibodies in the antiserum. Because bovine serum albumin shares some epitopes with human serum albumin in evolutionarily conserved regions of the protein, this anti-bovine serum albumin antiserum will also react with human serum albumin. Therefore, this antiserum will not be useful for distinguishing between bovine and human serum albumins.
Several approaches have been taken to overcome the specificity issue of polyclonal antisera. One is by absorbing the unwanted antibodies by passing the antiserum through a chromatography column of immobilized antigens (2). This method is tedious and frequently unable to completely remove the unwanted antibodies. Another approach is to isolate individual antibody-producing B cells and expand them in culture. However, like most normal untransformed cells, B cells do not survive in long-term culture.
To overcome the inability of B cells to survive in culture, one approach is to prepare a myeloma-B cell hybridoma. In 1847, Henry Bence-Jones discovered that patients with multiple myeloma, a lymphoid tumor, produced a large quantity of antibodies (3). B cells in these patients have become malignant and grow uncontrollably. Since the malignant B cells are derived from a single clone, they are identical and produce only a single type of antibody (i.e., a monoclonal antibody, or mAb). However, most of these myeloma cells produce antibodies of unknown specificities. In 1975, by fusing a myeloma cell to a B cell, Cesar Milstein and Georges Kohler succeeded in producing a hybridoma that can be cultured indefinitely in vitro and produces an unlimited number of monoclonal antibodies of known antigenic specificity (4). The rationale behind their approach is to combine the immortal properties of the myeloma cell and the antibody producing properties of the B cell. Their technique revolutionized antibody production and provides a powerful means for identification and purification of biological molecules using monoclonal antibodies.
Generally, preparing a monoclonal antibody requires several months. The general procedure includes the following steps:
- Immunization and screening of antibody titer
- Fusion of antibody-producing B cells and myeloma cells
- Selective growth of the hybridoma
- Screening the hybridomas for producing the desired monoclonal antibody
- Cloning by limiting dilution - a process whereby cells are diluted to a concentration to statistically allow for less than 1 cell to be added to the wells of a 96-well plate. Some wells will end up with 0 cells and some will have 1 cell. The wells with seeded with 1 cell will eventually grow into a monoclonal population of cells.
- Growth of the hybridoma and preparation of monoclonal antibody
This protocol focuses on the last step - growth of the hybridoma and preparation of the monoclonal antibody. The antibody is purified from the culture supernatant by ammonium sulfate precipitation (often referred to as salting out) - a commonly used method of removing proteins from a solution. Proteins in solution form hydrogen bonds, along with other hydrophilic interactions, with water through their exposed polar and ionic groups. When concentrations of small, highly charged ions (such as ammonium or sulfate) are added, these groups compete with the proteins for binding to water. This removes the water molecules from the protein and decreases its solubility, resulting in precipitation of the protein.
Procedure
Note: Sterile cell culture technique should be maintained when handling the hybridoma cells and the media in a sterile manner (e.g., in a biosafety cabinet) until the antibody purification steps.
1. Thawing frozen hybridoma cells
- Incubate the vial containing the frozen hybridoma cells in a 37°C water bath until just thawed (approximately 2 minutes).
- Add the thawed cells to a 15 mL conical tube containing 10 mL of complete RPMI (RPMI
Results
Using this protocol, we have obtained the following results with several different hybridomas:
Hybridoma: RB6-BC5 (rat anti-mouse Ly6C/Ly6G (Gr1) IgG2b, κ mAb)
OD280 - 1.103
(1.103/1.43)(20) = 15.42 mg/mL
Hybridoma: GK1.5 (rat anti-mouse CD4 IgG2b, κ mAb)
OD280 - 0.485
(0.485/1.43)(20) = 6.78 mg/
Application and Summary
The procedure outlined above is a simple, straight-forward way to purify monoclonal antibodies from hybridoma culture supernatant. It is important to remember, though, that the ammonium sulfate will precipitate other proteins that may be in the culture supernatant. Consequently, the antibody concentrations determined from the absorbance measurements are estimates. The user may wish to assess the purity of the dialyzed sample by running a small amount on an SDS-polyacrylamide gel. The mAb produced by a hybridoma, once pur
References
- Lipman NS, Jackson LR, Trudel LJ, Weis-Garcia F. Monoclonal versus polyclonal antibodies: distinguishing characteristics, applications, and information resources. ILAR Journal, 46 (3), 258-268 (2005).
- Arora S, Ayyar BV, O'Kennedy R. Affinity chromatography for antibody purification Methods Mol Biol. 1129, 497-516 (2014).
- Henry BJ. On a new substance occurring in the urine of a patient with mollities ossium. Philosophical Transactions of the Royal Society of London. 138, 55-62 (1848).
- Köhler G and Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity". Nature. 256, 495-497 (1975).
Skip to...
Videos from this collection:

Now Playing
Antibody Generation: Producing Monoclonal Antibodies Using Hybridomas
Immunology
43.6K Views

Flow Cytometry and Fluorescence-Activated Cell Sorting (FACS): Isolation of Splenic B Lymphocytes
Immunology
93.0K Views

Magnetic Activated Cell Sorting (MACS): Isolation of Thymic T Lymphocytes
Immunology
22.9K Views

ELISA Assays: Indirect, Sandwich, and Competitive
Immunology
238.7K Views

ELISPOT Assay: Detection of IFN-γ Secreting Splenocytes
Immunology
28.5K Views

Immunohistochemistry and Immunocytochemistry: Tissue Imaging via Light Microscopy
Immunology
79.0K Views

Immunofluorescence Microscopy: Immunofluorescence Staining of Paraffin-Embedded Tissue Sections
Immunology
53.9K Views

Confocal Fluorescence Microscopy: A Technique to Determine the Localization of Proteins in Mouse Fibroblasts
Immunology
43.2K Views

Immunoprecipitation-Based Techniques: Purification of Endogenous Proteins Using Agarose Beads
Immunology
87.8K Views

Cell Cycle Analysis: Assessing CD4 and CD8 T Cell Proliferation After Stimulation Using CFSE Staining and Flow Cytometry
Immunology
24.3K Views

Adoptive Cell Transfer: Introducing Donor Mouse Splenocytes to a Host Mouse and Assessing Success via FACS
Immunology
22.5K Views

Assay for Cell Death: Chromium Release Assay of Cytotoxic Ability
Immunology
151.4K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved