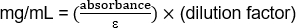항체 생성: 융합 세포를 사용한 단일 클론 항체 생성
Overview
출처: 프랜시스 V. 자아스타드1,2,휘트니 스완슨2,3,토마스 에스 그리피스1,2,3,4
1 미생물학, 면역학 및 암 생물학 대학원 프로그램, 미네소타 대학교, 미니애폴리스, MN 55455
2 면역학 센터, 미네소타 대학, 미니애폴리스, MN 55455
3 미네소타 대학교 비뇨기과, 미니애폴리스, MN 55455
4 Masonic 암 센터, 미네소타 대학, 미니애폴리스, MN 55455
다각성 항체는 항원 또는 여러 항원(1)의 상이한 항원 결정제에 대하여 지시된 항체의 집합으로 정의됩니다. 다각성 항체는 생물학적 분자를 식별하기위한 강력한 도구이지만, 한 가지 중요한 한계가 있습니다 - 항원 결정제를 공유하는 항원을 구별 할 수 없습니다. 예를 들어, 소 혈청 알부민이 동물을 예방 접종하는 데 사용될 때, 다른 표면이 있는 B 세포는 소 세럼 알부민에 대한 상이한 항원 결정제에 반응하게 된다. 그 결과 항체의 혼합물이 항혈구에 혼합된다. 소 세럼 알부민은 단백질의 진화적으로 보존된 영역에서 인간 혈청 알부민과 일부 에피토페를 공유하기 때문에, 이 안티소빈 세럼 알부민 안티세럼은 또한 인간 혈청 알부민과 반응할 것이다. 따라서,이 항세럼은 소와 인간의 혈청 알부민을 구별하는 데 유용하지 않습니다.
다발성 항세라의 특이성 문제를 극복하기 위해 몇 가지 접근법이 취해졌습니다. 하나는 고정된 항원의 크로마토그래피 컬럼을 통해 항혈을 전달함으로써 원치 않는 항체를 흡수함으로써(2)이다. 이 방법은 지루하고 자주 완전히 원치 않는 항체를 제거 할 수 없습니다. 또 다른 접근법은 개별 항체 생산 B 세포를 분리하고 배양에서 확장하는 것입니다. 그러나, 대부분의 일반적인 변환되지 않은 세포같이, B 세포는 장기 배양에서 살아남지 않습니다.
배양에서 살아남기 위해 B 세포의 무능력을 극복하기 위해, 한 가지 방법은 골수종-B 세포 혼종을 준비하는 것이다. 1847년, 헨리 Bence-Jones는 림프성 종양인 다발성 골수종을 가진 환자가 다량의 항체(3)를 생산한다는 것을 발견했습니다. 이 환자에 있는 B 세포는 악성되고 통제할 수 없는 성장합니다. 악성 B 세포는 단일 클론으로부터 파생되기 때문에, 그들은 동일하고 항체의 단일유형(즉,단일 클론 항체 또는 mAb)만 생성한다. 그러나, 이러한 골수종 세포의 대부분은 알 수 없는 특이성의 항체를 생산. 1975년, 골수종 세포를 B 세포에 융합시킴으로써, 세자르 밀스타인과 조지 콜러는 시험관내에서 무기한 배양될 수 있는 혼종 생성에 성공하여 알려진 항원 특이성의 무제한 의 단일클론 항체를 생산한다(4). 그들의 접근의 뒤에 근거는 골수종 세포의 불멸의 속성 및 B 세포의 생성 하는 항체를 결합 하는. 그들의 기술은 항체 생산을 혁명화하고 단일 클론 항체를 사용하여 생물학적 분자의 식별 및 정화를위한 강력한 수단을 제공합니다.
일반적으로 단일 클론 항체를 준비하는 것은 몇 달이 필요합니다. 일반 절차에는 다음 단계가 포함됩니다.
- 항체 티터의 예방 접종 및 선별
- 항체 생성 B 세포 및 골수종 세포의 융합
- 혼종의 선택적 성장
- 원하는 단일 클론 항체를 생산하기위한 혼종 스크리닝
- 희석을 제한하여 복제 - 세포가 통계적으로 96웰 플레이트의 우물에 첨가될 1세포 미만을 허용하도록 농도로 희석되는 과정. 일부 우물은 0 세포로 끝나고 일부는 1 개의 세포가 있습니다. 1 세포와 함께 씨를 뿌렸던 우물은 결국 세포의 단일 클론 인구로 성장할 것입니다.
- 단일 클론 항체의 하이브리드 종의 성장 및 준비
이 프로토콜은 마지막 단계에 초점을 맞추고 - 혼성종의 성장과 단일 클론 항체의 준비. 항체는 암모늄 황산염 강수량(종종 염화아웃이라고도 함)에 의해 배양 상수로부터 정제된다 - 용액에서 단백질을 제거하는 일반적으로 사용되는 방법. 용액의 단백질은 노출된 극지 및 이온 그룹을 통해 물과 함께 다른 친수성 상호 작용과 함께 수소 결합을 형성합니다. 작고 고압이 많은 이온(예: 암모늄 또는 황산염)의 농도가 추가되면, 이들 그룹은 물에 결합하기 위한 단백질과 경쟁한다. 이것은 단백질에서 물 분자를 제거하고 그것의 용해도를 감소시켜 단백질의 강수량을 초래합니다.
Procedure
참고: 멸균 세포 배양 기술은 항체 정화 단계까지 혼성종 세포 및 미디어를 멸균 방식으로(예를 들어, 생물안전 캐비닛에서)로 처리할 때 유지되어야 한다.
1. 동결 된 혼종 세포를 해동
- 37°C 수조에서 냉동 혼종 세포를 함유한 바이알을 해동(약 2분)까지 배양합니다.
- 해동 된 세포를 완전한 RPMI10 mL (RPMI는 10 % 태아 소 세럼, 100 U /mL 페니실린, 100 μg /mL 연쇄 상감, 1mM 나트륨 pyruvate, 1x 비 필수 아미노산, 50 μ-10 μm)를 포함하는 15 mL 원추형 튜브에 추가하십시오.
- 1200 RPM에서 5분 동안 원심분리기를 사용하여 오염된 동결 매체를 씻어낼 수 있습니다.
- 원심 분리 후 액체 상체를 버리고 5mL의 신선한 완전 RPMI로 셀 펠릿을 다시 분리하십시오. 이어서, 15mL 완전 RPMI
Results
이 프로토콜을 사용하여 다음과 같은 결과를 여러 가지 혼성종으로 얻었습니다.
하이브리드종: RB6-BC5 (쥐 안티 마우스 Ly6C/Ly6G (Gr1) IgG2b, θ mAb)
OD280 - 1.103
(1.103/1.43) (20) = 15.42 mg/mL
하이브리드종: GK1.5 (쥐 안티 마우스 CD4 IgG2b, θ mAb)
OD280 - 0.485
(0.4...
Application and Summary
위에서 설명한 절차는 혼종 배양 상수에서 단클론 항체를 정화하는 간단하고 직선적인 방법입니다. 하지만 황산암모늄은 문화상수퍼에 있을 수 있는 다른 단백질을 침전시킬 것이라는 점을 기억하는 것이 중요합니다. 따라서, 흡광도 측정에서 결정된 항체 농도는 추정치이다. 사용자는 SDS-폴리아크라이글라미드 젤에 소량을 실행하여 투석된 샘플의 순도를 평가할 수 있다. 이 방법론을 사용하여...
References
- Lipman NS, Jackson LR, Trudel LJ, Weis-Garcia F. Monoclonal versus polyclonal antibodies: distinguishing characteristics, applications, and information resources. ILAR Journal, 46 (3), 258-268 (2005).
- Arora S, Ayyar BV, O'Kennedy R. Affinity chromatography for antibody purification Methods Mol Biol. 1129, 497-516 (2014).
- Henry BJ. On a new substance occurring in the urine of a patient with mollities ossium. Philosophical Transactions of the Royal Society of London. 138, 55-62 (1848).
- Köhler G and Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity". Nature. 256, 495-497 (1975).
Tags
건너뛰기...
이 컬렉션의 비디오:

Now Playing
항체 생성: 융합 세포를 사용한 단일 클론 항체 생성
Immunology
43.5K Views

유세포 분석 및 형광 활성화 세포 분류 (FACS): 비장 B 림프구의 분리
Immunology
92.9K Views

자기 활성화 세포 분류 (MACS): 흉선 T 림프구 분리
Immunology
22.9K Views

ELISA 분석: 간접, 샌드위치 및 길항
Immunology
238.1K Views

ELISPOT 분석: IFN-γ 분비 비장세포 검출
Immunology
28.4K Views

면역 조직 화학 및 면역 세포 화학: 광학 현미경을 통한 조직 이미징
Immunology
78.8K Views

면역 형광 현미경 검사법: 파라핀이 내장 된 조직 절편의 면역 형광 염색
Immunology
53.8K Views

공 초점 형광 현미경: 쥐 섬유 아세포에서 단백질의 국소화를 결정하는 기술
Immunology
43.1K Views

면역침전반응 기반 기술: 아가로스 비즈를 사용한 내인성 단백질의 정제
Immunology
87.6K Views

세포주기 분석: 세포주기 CFSE 염색 및 유세포 분석을 사용한 자극 후 CD4 및 CD8 T 세포 증식 평가
Immunology
24.2K Views

입양 세포 전송: 기증자 쥐 비장 세포를 숙주 쥐에 도입 후 FACS를 통한 성공 평가
Immunology
22.3K Views

세포 사멸 분석: 세포 독성 능력의 크롬 방출 분석
Immunology
151.4K Views
Copyright © 2025 MyJoVE Corporation. 판권 소유