理想气体定律
Overview
资料来源: 实验室的博士 Andreas Züttel-瑞士联邦实验室材料科学与技术
理想气体定律描述最常见气体在附近环境条件下的行为和所有化学物质在稀释极限下的趋势。它在系统中,是三个可衡量宏观系统变量 (压力、 温度和体积) 与气体分子的数目之间的基本关系,因此是微观和宏观的宇宙之间必不可少的环节。
理想气体定律的历史可以追溯到17 世纪中叶时候的压力和体积的空气之间的关系被发现是成反比,证实了由罗伯特 · 波义耳和我们现在称之为 · 博伊尔的法律 (方程 1) 的表达式。
P 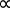 V-1 (方程 1)
V-1 (方程 1)
发表的作品,由雅克 · 查尔斯在 1780 年代,并被给予大量的气体和蒸气的约瑟夫 · 路易斯 · 吕报道于 1802 年,成立的绝对温度和气体体积的正比关系。这种关系称为查尔斯的法律 (方程 2)。
V 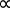 T (方程 2)
T (方程 2)
纪尧姆 · Amontons 通常归功于18 世纪初第一次发现固定体积内空气的压力与温度的关系。这项法律还向许多其他气体由约瑟夫 · 路易斯 · 吕19 世纪初,因此也被称为 Amontons 的法律或同性恋者 Lussac 法,指出方程 3中所示。
P 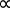 T (方程 3)
T (方程 3)
在一起,可以结合这三组关系给关系方程 4中。
V 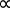 T (方程 4)
T (方程 4)
最后,在 1811 年,它提出了阿米阿佛加德罗任何两种气体,在相同的体积和在相同的温度和压力,举行包含相同数目的分子。这导致所有气体可以通过一个共同的常数,理想气体常数 R,即独立的气体的性质进行都描述的结论。这就是所谓的理想气体定律 (方程 5)。1,2
PV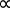 T (方程 5)
T (方程 5)
Procedure
1.测量体积的样品
- 仔细清洁样品和干燥它。
- 一个高分辨率的量筒装满足够蒸馏水覆盖样品。请注意初始音量
- 掉进了水的样本,并注意体积的变化。这是样本体积,V。
- 删除示例然后擦干。注意: 或者,测量样品的侧 length(s),并计算其体积使用几何。
2.负载平衡中的示例
- 挂在磁悬浮平衡样品。
- 安装压力/温度室围绕样品。
- 撤离的示例环境和加氢气,到 1 栏。
- 测量样品重量在 1 酒吧和室温下,w00。
3.测量样品重量为常温压力的函数
- 增加或减少在 Pi0样品环境压力。
- 允许样品环境平衡。
- 测量样本,wi0的重量。
- 3.1-3.3 重复无数次。
.css-f1q1l5{display:-webkit-box;display:-webkit-flex;display:-ms-flexbox;display:flex;-webkit-align-items:flex-end;-webkit-box-align:flex-end;-ms-flex-align:flex-end;align-items:flex-end;background-image:linear-gradient(180deg, rgba(255, 255, 255, 0) 0%, rgba(255, 255, 255, 0.8) 40%, rgba(255, 255, 255, 1) 100%);width:100%;height:100%;position:absolute;bottom:0px;left:0px;font-size:var(--chakra-fontSizes-lg);color:#676B82;}
Results
Application and Summary
References
- Zumdahl, S.S., Chemical Principles. Houghton Mifflin, New York, NY. (2002).
- Kotz, J., Treichel, P., Townsend, J. Chemistry and Chemical Reactivity. 8th ed. Brooks/Cole, Belmont, CA (2012).
- Rouquerol, F., Rouquerol, J., Sing, K.S.W., Llewellyn, P., Maurin, G. Adsorption by Powders and Porous Solids: Principles, Methodology and Applications.Academic Press, San Diego, CA. (2014).
Tags
跳至...
此集合中的视频:

Now Playing
理想气体定律
General Chemistry
79.0K Views

常见的实验室玻璃器皿和用途
General Chemistry
658.3K Views

溶液和浓度
General Chemistry
275.1K Views

固体和液体的密度测定
General Chemistry
556.8K Views

确定在水溶液中的质量百分组成
General Chemistry
383.8K Views

确定经验公式
General Chemistry
183.7K Views

确定离子化合物的溶度积的规则
General Chemistry
141.6K Views

使用 pH 计
General Chemistry
346.7K Views

滴定法简介
General Chemistry
425.4K Views

平衡常数的分光光度法测定
General Chemistry
158.7K Views

Le Châtelier 原则
General Chemistry
265.8K Views

凝固点降低,以确定一种未知的化合物
General Chemistry
160.8K Views

确定率法律和秩序的反应
General Chemistry
196.3K Views

用焓差扫描量热法测量的变化
General Chemistry
44.7K Views

配合化学物
General Chemistry
91.7K Views
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。
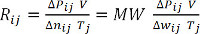 (方程 9)
(方程 9)