Method Article
En Vivo Transferencia de genes a células de Schwann en el nervio ciático de roedores mediante electroporación
En este artículo
Resumen
Here, we present an in vivo technique for gene transfer to Schwann cells (SCs) in the rodent sciatic nerve. This simple technique is useful for investigating signaling mechanisms involved in the development and maintenance of myelinating SCs.
Resumen
The formation of the myelin sheath by Schwann cells (SCs) is essential for rapid conduction of nerve impulses along axons in the peripheral nervous system. SC-selective genetic manipulation in living animals is a powerful technique for studying the molecular and cellular mechanisms of SC myelination and demyelination in vivo. While knockout/knockin and transgenic mice are powerful tools for studying SC biology, these methods are costly and time consuming. Viral vector-mediated transgene introduction into the sciatic nerve is a simpler and less laborious method. However, viral methods have limitations, such as toxicity, transgene size constraints, and infectivity restricted to certain developmental stages. Here, we describe a new method that allows selective transfection of myelinating SCs in the rodent sciatic nerve using electroporation. By applying electric pulses to the sciatic nerve at the site of plasmid DNA injection, genes of interest can be easily silenced or overexpressed in SCs in both neonatal and more mature animals. Furthermore, this in vivo electroporation method allows for highly efficient simultaneous expression of multiple transgenes. Our novel technique should enable researchers to efficiently manipulate SC gene expression, and facilitate studies on SC development and function.
Introducción
The rapid transmission of sensory and motor information in the peripheral nervous system is permitted by the myelin sheath, which is formed by myelinating Schwann cells (SCs)1. Insulation of axons by the myelin sheath enables saltatory conduction, which increases the speed of nerve impulses. In disorders in which the development or maintenance of the myelin sheath is impaired, nerve conduction speed is reduced. This results in neuropathy involving motor and sensory dysfunction. Although there are many studies on the molecular mechanisms of myelination and demyelination in the peripheral nervous system, the roles of the numerous proteins involved in these processes remain unclear.
To study the molecular mechanisms of SC myelination/demyelination in vivo, genetic approaches have been used to modify gene expression in animals. A powerful approach is the use of knockout/knockin or transgenic animals. However, the generation of these animals is expensive and time consuming. For SC-specific gene manipulation, crossing floxed strains with Cre mice or other conditional gene expression methods are necessary. This again is laborious and time intensive. In recent years, a cutting-edge genetic technology, the CRISPR-Cas9 system, has made the generation of genetically modified mice much quicker (about 4 weeks)2,3, but this method is hindered by target sequence limitations, and suffers from off-target effects. As an alternative method, viral vector-mediated gene transfer is a faster and easier method of achieving gene transfer into SCs in vivo4-6. Indeed, the generation of viral vectors is less expensive, and takes a shorter time (within a few weeks), and gene manipulation of SCs can be achieved by simply injecting engineered viral vectors, such as adenoviral vectors, adeno-associated viral (AAV) vectors, and lentiviral vectors, into the sciatic nerve. Because these viral vectors have different characteristics, users have to choose the one best suited for their purpose. Adenoviral vectors infect axons and SCs in both young and mature sciatic nerves. In particular, adenoviral vectors have higher selectively for non-myelinating SCs than myelinating SCs. Adenoviruses can cause immune responses, and accordingly, immunodeficient strains should be used5. AAV vectors are currently the most widely used viral vectors, and allow in vivo gene transfer with lower toxicity7. AAV can transduce both axons and SCs by direct injection into the nerve fibers8,9. However, AAV-mediated protein expression usually requires 3 weeks or longer to reach maximum levels7,9. Therefore, it is difficult to analyze myelination, which actively progresses during the two week postnatal period. Lentiviral vectors have higher selectively for myelinating SCs than non-myelinating SCs, and do not have toxic effects on sciatic nerves. However, lentiviral vectors do not infect SCs in more mature nerves5, and therefore are unsuitable for analyzing events such as the demyelination process.
Electroporation is another faster and easier approach to achieve in vivo gene transfer. It has been reported that in vivo transfection of SCs can be achieved when electroporation is applied to transected rat sciatic nerves10. However, because this method requires nerve transection for gene delivery, the application is limited to the analysis of the damaged nerves. Here, we describe an alternative method that allows the delivery of transgenes into myelinating SCs in intact rat sciatic nerves using electroporation11. This method requires plasmid construction, which can usually be completed within a week. Then, by simply delivering electric pulses to the site on the sciatic nerve where the plasmid DNA was injected, highly selective transfection of myelinating SCs can be achieved in neonatal as well as in more mature animals. By electroporating multiple plasmids, simultaneous expression of a variety of genes can be easily achieved. The ability to simultaneous express multiple molecules, such as signaling proteins, short-hairpin RNAs (shRNAs) and functional probes, is crucial for investigating complex processes such as myelination and demyelination. The novel in vivo electroporation method described in this paper will be a powerful tool, allowing researchers to analyze the function of a multitude of molecules and their interactions in myelinating SCs.
Protocolo
El uso de ratas para la investigación estaba en conformidad con las directrices establecidas por el Comité de Protección de los Animales de la Universidad de Tokio.
1. Preparación de ADN plásmido
- Generar plásmidos de ADN para la electroporación in vivo por subclonación del cDNA o secuencia shRNA en un plásmido de expresión para células de mamíferos 12. Use un citomegalovirus temprano inmediato de potenciador y de pollo β-actina de promotor de fusión (CAG) promotor impulsado plásmido 13, ya que permite la expresión fuerte y estable. Para la expresión de shRNAs bajo el control de un promotor de CAG, utilizar un sistema de casete de shRNA basado en mir30 para subclonar el shRNA 14.
- Se purifica ADN de plásmido con un kit maxi-prep de acuerdo con las instrucciones del fabricante, y resuspender el DNA con solución salina tamponada con HEPES (NaCl 140 mM, 0,75 mM Na 2 HPO 4, HEPES 25 mM; pH 7,40). Ajustar la concentración de DNA a ≥ 4 g / l.
- Preparar la solución de ADN del plásmido a una concentración de 4 g / l, y añadir una cantidad mínima de tinte verde rápido (concentración final de 0,01%) para marcar el sitio de la inyección. Cuando se requiere la electroporación simultánea de múltiples plásmidos, ajustar la concentración total de la solución de ADN plasmídico a 4 g / l.
Nota: La composición óptima de ADN de plásmido se debe determinar de acuerdo con la eficiencia de la transfección de cada plásmido.
2. La esterilización de instrumentos quirúrgicos y solución salina
- instrumentos quirúrgicos autoclave y la solución de NaCl 0,9%.
3. Preparación de la micropipeta de vidrio
- Tire de pipetas de vidrio utilizando un extractor de pipeta. Cortar la punta de la pipeta a un diámetro de 30 a 50 micras. Utilice los siguientes parámetros: Calor, 600; Velocity, 50; Tiempo, 75.
4. Cirugía animal, la inyección de ADN y la electroporación
Nota: Un excesovista de este paso es descrito en la Figura 1. Aunque se describen el procedimiento de crías de rata aquí, el método es también aplicable a animales más maduros utilizando el mismo procedimiento.
- Anestesiar la rata con isoflurano en el cuadro de inducción hasta que el animal se inmoviliza mediante el ajuste del flujo de oxígeno a 0,4 L / min y la concentración de isoflurano al 4% (vol / vol). Realizar dedo pellizcar para confirmar la anestesia adecuada.
- Ponga la rata en la precalentado más caliente bajo un microscopio binocular, y mantener la anestesia mediante la administración de isoflurano forma continua a través de la mascarilla. Ajuste el flujo de oxígeno a 0,2 L / min y la concentración de isoflurano al 2% (vol / vol). Aplique las gotas para evitar la sequedad de los ojos si los ojos del animal están abiertas.
- Fijar las piernas con cinta quirúrgica.
- Limpiar la piel en la cara posterior del muslo con povidona yodada, y hacer una incisión con un bisturí.
Nota: Afeitado áreas quirúrgicas si las áreas quirúrgicas están cubiertas con haire. - Exponer el nervio ciático mediante la creación de una abertura entre los músculo cuádriceps femoral y el músculo bíceps femoral con agujas de coser.
- Humedecer el nervio con una solución de NaCl al 0,9%. Absorber el exceso de agua con papel libre de pelusas.
- Insertar la base de una micropipeta de vidrio en tubo flexible, y llenar la cantidad adecuada de solución de ADN (por lo menos un microlitro) en la micropipeta aspirando suavemente.
- Levante el nervio expuesto tirando suavemente de la parte distal del nervio usando una aguja.
Nota: No aplicar tensión al nervio para minimizar el estrés mecánico. - Insertar la micropipeta de vidrio en el sitio distal sobre el nervio, e inyectar la solución de ADN por la aplicación de presión (es decir, por soplado en el extremo abierto del tubo flexible). Inyectar la solución de ADN hasta que aparezca el nervio verde (1 l como máximo). Debido a la inserción frecuente de la micropipeta puede dañar el nervio, no inserte la micropipeta más de dos veces.
- Coloque un TWEezer de tipo electrodo de platino de aproximadamente 1-2 mm de distancia del nervio. Llenar el hueco entre el electrodo y el nervio con una solución de NaCl al 0,9%.
Nota: No sostenga el nervio con el electrodo para evitar el estrés mecánico sobre el nervio. - Aplicar pulsos eléctricos a la zona de la inyección utilizando un electroporador con el electrodo. Después de la primera serie de impulsos, invierta el electrodo y aplicar otra serie de impulsos. Utilice los siguientes parámetros: tensión, 50 V; duración del pulso, a 5 mseg; intervalo de impulso, 100 mseg; número de impulsos, 4 veces.
- Limpiar el sitio de la electroporación con una solución de NaCl al 0,9%.
- Repita los pasos 4.4 a 4.11 en el nervio ciático contralateral.
5. Post-electroporación
- Cerrar las incisiones con pegamento de cianoacrilato.
- Después de secar el pegamento, limpiar la herida con povidona-yodo.
- Liberar al cachorro de la máscara de la cara. Calentar el cachorro en un calentador al menos durante una hora con el fin de permitir que se recupere completamente de la anestesia. reo deje desatendido el cachorro hasta que se haya recuperado el conocimiento suficiente.
- Después de la recuperación de la anestesia, devolver el cachorro a la rata madre. No devuelva el cachorro hasta que esté completamente recuperado.
6. Post-cirugía
- Casa las crías de ratas en la jaula hasta la realización de los experimentos 11 (ver ejemplos en la Figura 3). Administrar carprofeno (5 mg / kg; ip), un fármaco no esteroide anti-inflamatorio, o buprenorfina (0,1 mg / kg, sc), un analgésico opioide, si se requiere.
Nota: Si la cría de rata no crece bien o se observa inflamación alrededor del sitio de la cirugía, excluir a los animales a partir de los experimentos.
Resultados
Un ejemplo de un nervio ciático transfectadas con la proteína fluorescente roja (RFP) expresando plásmido se muestra en la Figura 2A. Células que muestran la morfología bipolar, una característica de SCs, se transfectaron escasamente con RFP. No se detectó fluorescencia de RFP en los axones. Por lo general, encontramos ~ 100 SC transfectadas en cada nervio. Esta eficiencia de transfección parece similar a la eficacia de infección SC in vivo utilizando vectores lentivirales 4.
Experimentos de inmunotinción mostraban que la mayoría (~ 96%) de las células RFP-positiva en P7 co-etiquetados para S100, un marcador SC (Figura 2B), y 91% de las células RFP-positiva en P14 co-etiquetados para MBP, un marcador myelinating SC (Figura 2C), lo que sugiere que la transferencia génica mediante electroporación es altamente selectivo para myelinating SCs.
Introducción de múltiples genes en las SC in vivo será de gran utilidad para la investigación de los mecanismos de mielinización / desmielinización. Una ventaja importante del método de la electroporación in vivo descrito aquí es la capacidad de transferir genes múltiples con un procedimiento simple. La figura 2D muestra una imagen representativa de un nervio ciático transfectadas con una mezcla de GFP y RFP plásmidos que expresan el uso de la electroporación in vivo. Alrededor del 97% de los SCs fueron GFP y RFP doble positivo, lo que sugiere que la entrega altamente eficiente de múltiples genes puede conseguirse simplemente electroporación mezclas de múltiples plásmidos.
En roedores, la mielinización inicia alrededor del nacimiento, lo que aumenta drásticamente durante las dos primeras semanas después del nacimiento, y luego disminuye gradualmente. Por lo tanto, por la manipulación genética de las SC durante estas ventanas de tiempo de desarrollo, los mecanismos subyacentes a estos diferentes etapas de la mielinización se pueden aclarar. lentiviral vectores son una buena herramienta para unanalizado mielinización, en particular, ya que tienen una toxicidad mínima, pero los lentivirus infectan solamente neonatal nervios ciáticos 5,6. En comparación, la transferencia de genes mediada por electroporación funciona bien cuando la transfección se lleva a cabo en P3 (Figura 2E, arriba) o en P14 (Figura 2E, abajo).
Aquí se describen las aplicaciones del nuevo método de electroporación in vivo. La figura 3A muestra imágenes de microscopía óptica de expresión de GFP-SC myelinating en las distintas etapas del desarrollo (P7, P14, P21 y P31). Por análisis microscópico de luz, cambios en los parámetros morfológicos, como la longitud y el diámetro, se pueden evaluar. Tenga en cuenta que estos parámetros tienen valores similares en comparación con los nervios periféricos de rata intacta 15,16, lo que sugiere que los nervios electroporadas se desarrollan sin efectos perjudiciales significativos. La Figura 3B muestra una imagen de microscopía electrónica de LacZ-expressing myelinating SC. En este caso, LacZ fue utilizado como un marcador de expresión. β-galactosidasa tinción utilizando Bluo-gal, un sustrato insoluble en etanol, permite el análisis de la estructura de la mielina de las SC transfectadas mediante microscopía electrónica de 11,17. En estos experimentos, el papel de las moléculas de señalización puede ser examinado por el silenciamiento o aumentar su expresión, lo que permite el análisis de la pérdida de función o los efectos de ganancia de función. Además del análisis de tejido fijado, transferencia génica in vivo mediada por electroporación también se puede aplicar a vivir experimentos de imagen. Por ejemplo, la figura 3C muestra una myelinating SC co-expresión de G-GECO1.1 18, un indicador fluorescente verde citosólica de Ca2 +, y 19 R-GECO1mt, un indicador fluorescente de color rojo mitocondrial de Ca2 +. Mediante la expresión de estos indicadores, hemos identificado una vía de señalización que controla citosólica y mitocondrial de Ca 2 + concentraciones en myelinating SC . Por lo tanto, el presente método se puede utilizar para estudiar una variedad de mecanismos de señalización, en particular cuando las sondas fluorescentes codificadas genéticamente están disponibles para detectar las señales de interés.
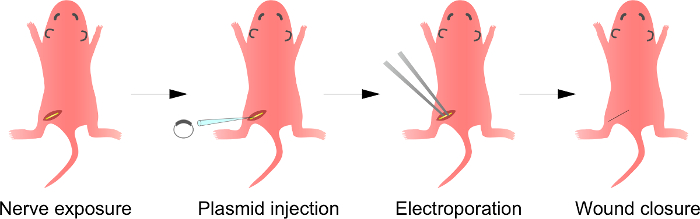
Figura 1:. Esquemática de la i n electroporación in vivo Método En primer lugar, el nervio ciático de la rata anestesiada se expone. En segundo lugar, el plásmido de ADN se inyecta en el nervio ciático. En tercer lugar, los impulsos eléctricos se entregan al sitio de inyección a través del electrodo en forma de fórceps. Por último, la herida se cierra con pegamento. Este procedimiento se puede repetir en el nervio contralateral. Por favor, haga clic aquí para ver una versión más grande de esta figura.
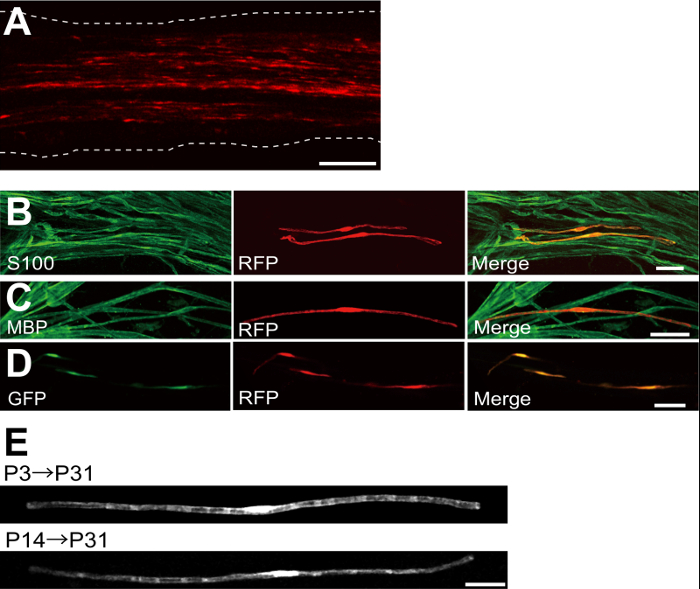
Figura 2: Los resultados representativos en nervios ciáticos transfectadas (A) Una imagen representativa de un nervio ciático transfectadas.. El nervio se transfectadas con el plásmido que expresa al RFP-P3, y se fija en P7. (B) Una imagen representativa de una célula RFP-transfectadas en P7 mostrando colocalización con S100, un marcador SC. (C) Una imagen representativa de un nervio ciático RFP-transfectadas en P14 mostrando colocalización con MBP, un marcador myelinating SC. (D) Una imagen representativa de un nervio ciático cotransfectadas con plásmidos GFP y RFP-expresión. SC transfectadas expresan simultáneamente GFP y RFP. (E) Una imagen de myelinating SCS P31 transfectadas en P3, cuando comienza la mielinización (arriba), y una imagen de las SC myelinating en P31 transfectadas en P14, cuando la mayoría de grandes axones mielinizados convierten (abajo), lo que sugiere que transfreflejo de las SC myelinating se puede lograr no sólo en los nervios neonatal, sino también en los nervios más maduros. Barras de escala = 200 m (A); 50 micras (BE). Esta cifra se modificó a partir de nuestra publicación anterior 11. Haga clic aquí para ver una versión más grande de esta figura.
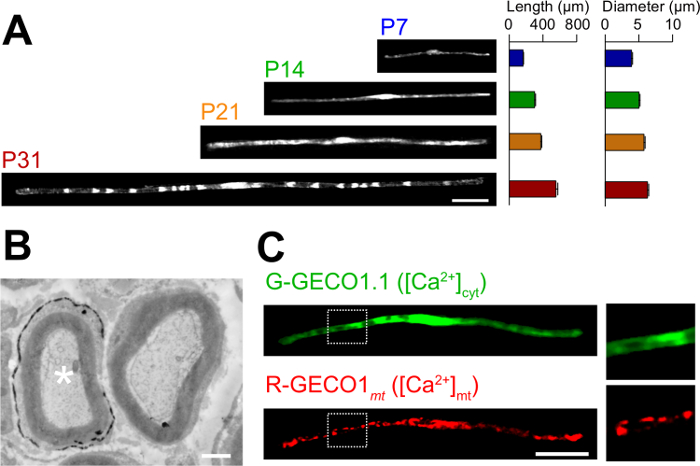
Figura 3: Aplicación de electroporación in vivo (a) un análisis microscópico de luz del desarrollo de myelinating SC.. Los nervios ciáticos se sometieron a electroporación con el plásmido que expresa GFP en P3, y se fijaron en las diversas etapas del desarrollo (P7, P14, P21 y P31). Imágenes representativas de las SC positivas para GFP se muestran a la izquierda. La longitud media y el diámetro se resumen como media ± SEM (n = 30 - 47 desde el de 3 nervios) a la derecha. La longitud y el diámetro de myelinating castas aumenta a medida que avanza el desarrollo. (B) Un electrón imagen microscópica de un nervio ciático transfectadas con un plásmido que codifica LacZ. A transfectadas SC (asterisco blanco, izquierda) se finamente etiquetado con precipitados del producto de reacción β-galactosidasa. (C) Una imagen de un SC cotransfected con G-GECO1.1, un indicador fluorescente verde citosólica de Ca2 +, y R-GECO1mt, un indicador fluorescente de color rojo mitocondrial de Ca2 +. Las regiones dentro de los rectángulos de puntos blancos se muestran ampliada en los paneles de la derecha. Barras de escala = 50 micras (A y C); 1 m (B). Esta cifra se modificó a partir de nuestra publicación anterior 11. Haga clic aquí para ver una versión más grande de esta figura.
Discusión
In this paper, we describe a simple and efficient method that allows in vivo gene transfer to myelinating SCs in the rat sciatic nerve using electroporation. This method allows highly selective gene expression in myelinating SCs by simply applying electric pulses to the plasmid DNA-injected sciatic nerve. Because the molecular mechanisms of myelination and demyelination in the peripheral nervous system remain unclear, the present in vivo electroporation method will be a powerful tool to clarify the roles of multiple genes of interest in living animals.
A critical requirement of this method is to keep damage to the nerve during surgery to a minimal level. Should surgical damage cause excessive inflammation, the sciatic nerve may degenerate. To avoid this, one must conduct surgery with extreme care, so as to not damage the blood vessels around the nerve. Mechanical stress to the nerve during the surgery can also be a cause of nerve damage. To minimize mechanical stress, lifting the exposed nerve should be done as gently as possible, and the tweezer-type electrode should be placed close to the nerve without contact. Furthermore, electrical pulses that are too strong can cause undesirable large leg movement, which leads to mechanical stress, or can burn the nerve. If significant damages are observed in the nerves, we recommend reducing the electrical pulse intensities or placing the electrode further away from the nerve.
In our present protocol, CAG promoter-driven plasmids were used as expression vectors. CAG promoter-driven plasmids allow high levels of gene expression in myelinating SCs in vivo. We also have tried a CMV promoter, another widely used universal promoter for mammalian gene expression, but expression of the gene product was very weak. This is consistent with previous results, in which electroporation-mediated transfection was conducted in the embryonic brain20. Therefore, we recommend using CAG promoter-driven plasmids for the in vivo electroporation method.
Because axonal signaling is a key factor in myelination/demyelination21, gene modification in neurons is also important. However, delivery of transgenes using our in vivo electroporation method is limited to SCs. It has been reported that gene delivery into sciatic nerve axons can be achieved when in vivo electroporation is applied to dorsal root ganglion (DRG) neurons in adult rats22. This suggests that delivery of plasmid DNA to the cell body is likely to be critical for in vivo transfection of peripheral axons. Thus, to examine the involvement of axonal molecules in myelination/demyelination, researchers should use neuron-specific genetic methods such as genetically modified animals, neuron-specific viral vectors, or in vivo electroporation to DRG neurons.
Compared with current methods, such as the generation of genetically modified animal lines23 and delivery of transgenes by viral vectors4-6, gene modification of SCs by in vivo electroporation is simpler. This method only requires several days for plasmid DNA construction and one day for electroporation surgery. Plasmid DNA construction does not require a biohazard room that is usually essential for viral vector handling. In addition, one of the advantages of the electroporation method is the capacity for simultaneous expression of multiple gene products using a simple protocol. Our novel technique will be useful for analyzing the interaction of a variety of signaling molecules involved in myelination and demyelination. In particular, by permitting the cotransfection of a number of different intracellular fluorescent probes, our method should be a powerful tool for investigating intracellular signaling dynamics in SCs using live imaging experiments.
Divulgaciones
The authors declare that they have no competing financial interests.
Agradecimientos
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology to M.I. (21229004 and 25221304).
Materiales
| Name | Company | Catalog Number | Comments |
| Genopure Plasmid Maxi Kit | Roche | 03 143 422 001 | Plasmid DNA purification kit |
| Fast Green CFC | WAKO | 069-00032 | Dye for DNA injection |
| GC 150T-10 | HARVARD APPARATUS | 30-0062 | Glass capillary |
| Suction tubing | Drummond | 05-2000-00 | Suction tubing for micro injection |
| MODEL P-97 | SUTTER INSTRUMENT CO. | Micropipette puller | |
| CUY21 Single Cell | BEX | Electroporator CUY21 Single Cell | Pulse generator |
| Electric warmer | KODEN | CAH-6A | Warmer during the surgery |
| Isofluolane | Mylan | 1119701G1076 | Anesthetic |
Referencias
- Nave, K. A., Werner, H. B. Myelination of the nervous system: mechanisms and functions. Annu Rev Cell Dev Biol. 30, 503-533 (2014).
- Wang, H., et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 153, 910-918 (2013).
- Yang, H., Wang, H., Jaenisch, R. Generating genetically modified mice using CRISPR/Cas-mediated genome engineering. Nat Protoc. 9, 1956-1968 (2014).
- Cotter, L., et al. Dlg1-PTEN interaction regulates myelin thickness to prevent damaging peripheral nerve overmyelination. Science. 328, 1415-1418 (2010).
- Gonzalez, S., Fernando, R. N., Perrin-Tricaud, C., Tricaud, N. In vivo introduction of transgenes into mouse sciatic nerve cells in situ using viral vectors. Nat Protoc. 9, 1160-1169 (2014).
- Ozcelik, M., et al. Pals1 is a major regulator of the epithelial-like polarization and the extension of the myelin sheath in peripheral nerves. J Neurosci. 30, 4120-4131 (2010).
- Daya, S., Berns, K. I. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 21, 583-593 (2008).
- Glatzel, M., et al. Adenoviral and adeno-associated viral transfer of genes to the peripheral nervous system. Proc Natl Acad Sci U S A. 97, 442-447 (2000).
- Homs, J., et al. Schwann cell targeting via intrasciatic injection of AAV8 as gene therapy strategy for peripheral nerve regeneration. Gene Ther. 18, 622-630 (2011).
- Aspalter, M., et al. Modification of Schwann cell gene expression by electroporation in vivo. J Neurosci Methods. 176, 96-103 (2009).
- Ino, D., et al. Neuronal Regulation of Schwann Cell Mitochondrial Ca(2+) Signaling during Myelination. Cell Rep. 12, 1951-1959 (2015).
- Struhl, K. Chapter 3; Subcloning of DNA fragments. Curr Protoc Mol Biol. , Unit3 16 (2001).
- Niwa, H., Yamamura, K., Miyazaki, J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 108, 193-199 (1991).
- Chang, K., Elledge, S. J., Hannon, G. J. Lessons from Nature: microRNA-based shRNA libraries. Nat Methods. 3, 707-714 (2006).
- Schlaepfer, W. W., Myers, F. K. Relationship of myelin internode elongation and growth in the rat sural nerve. J Comp Neurol. 147, 255-266 (1973).
- Webster, H. D. The geometry of peripheral myelin sheaths during their formation and growth in rat sciatic nerves. J Cell Biol. 48, 348-367 (1971).
- Weis, J., Fine, S. M., David, C., Savarirayan, S., Sanes, J. R. Integration site-dependent expression of a transgene reveals specialized features of cells associated with neuromuscular junctions. J Cell Biol. 113, 1385-1397 (1991).
- Zhao, Y., et al. An expanded palette of genetically encoded Ca(2)(+) indicators. Science. 333 (2), 1888-1891 (2011).
- Suzuki, J., et al. Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA. Nat Commun. 5, 4153 (2014).
- Tabata, H., Nakajima, K. Labeling embryonic mouse central nervous system cells by in utero electroporation. Dev Growth Differ. 50, 507-511 (2008).
- Taveggia, C., Feltri, M. L., Wrabetz, L. Signals to promote myelin formation and repair. Nat Rev Neurol. 6, 276-287 (2010).
- Saijilafu, E. M., Hur, F. Q., Zhou, Genetic dissection of axon regeneration via in vivo electroporation of adult mouse sensory neurons. Nat Commun. 2, 543 (2011).
- Tanaka, Y., Hirokawa, N. Mouse models of Charcot-Marie-Tooth disease. Trends Genet. 18, S39-S44 (2002).
Reimpresiones y Permisos
Solicitar permiso para reutilizar el texto o las figuras de este JoVE artículos
Solicitar permisoThis article has been published
Video Coming Soon
ACERCA DE JoVE
Copyright © 2025 MyJoVE Corporation. Todos los derechos reservados