Purification des composés par recristallisation
Vue d'ensemble
Source : Laboratoire du Dr Jimmy Franco - Merrimack College
Recristallisation est une technique utilisée pour purifier les composés solides. 1 solides ont tendance à être plus solubles dans les liquides chauds que dans les liquides froids. Au cours de la recristallisation, un composé solide impur est dissous dans un liquide chaud jusqu'à ce que la solution est saturée, et le liquide puis laisser refroidir. 2 le composé devrait alors se forger cristaux relativement purs. Idéalement, toutes les impuretés présentes resteront dans la solution et ne figureront pas dans les cristaux en pleine croissance (Figure 1). Les cristaux peuvent alors être retirés de la solution par filtration. Pas tout le composé est recouvrable — certains resteront dans la solution et sera perdue.
Recristallisation n'est pas généralement considérée comme une technique de séparation ; Il s’agit plutôt d’une technique de purification dans lequel une petite quantité d’impureté est éloignée d’un composé. Toutefois, si les propriétés de solubilité des deux composés sont suffisamment différentes, recristallisation permet de les séparer, même s’ils sont présents en quantités presque égales. Recristallisation fonctionne mieux lorsque la plupart des impuretés ont déjà été enlevées par une autre méthode, comme la chromatographie sur colonne ou extraction.
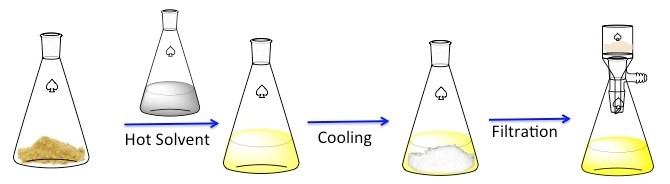
La figure 1. Du régime général de recristallisation.
Principles
Une recristallisation réussie dépend du choix approprié de solvant. Le composé doit être soluble dans le solvant chaud et insoluble dans les solvants même quand il fait froid. Dans le but de recristallisation, envisager 3 % p/v de la ligne de démarcation entre solubles et insolubles : si 3 g d’un composé se dissout dans 100 mL de solvant, il est considéré comme soluble. Dans le choix d’un solvant, plus la différence entre la solubilité chaude et froide solubilité, plus le produit récupérable de recristallisation.
La vitesse de refroidissement détermine la taille et la qualité des cristaux : refroidissement rapide favorise les petits cristaux, et un refroidissement lent favorise la croissance des cristaux de grandes et généralement plus pur. Le taux de recristallisation est habituellement maximale à environ 50 ° C au-dessous du point de fusion de la substance ; la formation maximale des cristaux se produit à environ 100 ° C au-dessous du point de fusion.
Bien que les termes « cristallisation » et « recristallisation » sont parfois utilisés de façon interchangeable, ils se réfèrent techniquement à différents processus. La cristallisation se réfère à la formation d’un nouveau produit insoluble par une réaction chimique ; ce produit précipite ensuite par la solution de réaction comme un solide amorphe contenant beaucoup d’impuretés piégés. Recristallisation n’implique pas une réaction chimique ; le produit brut est simplement dissous dans la solution, et puis les conditions sont modifiées pour permettre les cristaux à reformer. Recristallisation donne un produit final plus pur. Pour cette raison, les procédures expérimentales qui produisent un produit solide par cristallisation normalement incluent une étape finale recristallisation pour donner le composé pur.
Procédure
Effectuez toutes les opérations sous une hotte pour prévenir l’exposition aux vapeurs de solvants.
1. sélection d’un solvant
- Placer 50 mg de l’échantillon (N-bromosuccinimide) dans un erlenmeyer.
- Ajouter 0,5 mL de point d’ébullition du solvant (eau). Si l’échantillon se dissout complètement, la solubilité dans le solvant froid est trop élevée pour être un solvant bonne recristallisation.
- Si l’échantillon ne se dissout pas dans le solvant froid, faire chauffer le tube à essai jusqu'à ébullition du solvant.
- Si l’échantillon n’a pas complètement dissous à ce stade, ajouter plus de solvant bouillante goutte-à-goutte, jusqu'à ce que tous le solide se dissout. Si cela prend plus de 3 mL pour dissoudre l’échantillon dans le solvant à chaud, la solubilité dans ce solvant est probablement trop faible pour rendre un solvant bonne recristallisation.
- Si le premier choix du solvant n’est pas un solvant de bonne recristallisation, essayer d’autres. Si un solvant unique qui fonctionne est introuvable, essayez un système de deux solvants.
- Si vous ne trouvez pas un seul système de solvant approprié, puis une paire de solvant peut être nécessaire. Lorsqu’on veut identifier une paire de solvant, il y a plusieurs facteurs clés dont 1) le premier solvant devrait facilement dissoudre le solide. 2) le second solvant doit être miscible avec le solvant dest 1, mais ont une beaucoup plus faible solubilité pour le soluté.
- En règle générale « aime aime dissoudre », ce qui signifie que les composés polaires ont tendance à être soluble dans les solvants polaires et non polaires composés est souvent plus des composés non polaires solubles.
- Paires de solvants communs (tableau 1)
- Assurez-vous que le solvant a un point d’ébullition d’au moins 40 ° C, donc il y a une différence de température raisonnable entre le point d’ébullition du solvant et solvant à température ambiante.
- Veiller à ce que le solvant a environ 120 ° C, un point d’ébullition ci-dessous n’est plus facile d’éliminer les dernières traces de solvant des cristaux.
- Assurez-vous également que le point d’ébullition du solvant est plus bas que le point de fusion du composé, alors le composé forme des cristaux solides plutôt qu’une huile insoluble.
- Confirmer que les impuretés sont insolubles dans le solvant chaud (donc ils peuvent être chaud-filtrés, une fois que le composé est dissous) ou soluble dans le solvant froid (pour qu’ils restent dissous durant tout le processus).
2. dissoudre l’échantillon dans un solvant chaud
- Placez le composé à être recristallisée dans un erlenmeyer. Il s’agit d’un meilleur choix qu’un bécher, étant donné que les parois en pente aident piège vapeurs de solvants et ralentissent la vitesse d’évaporation.
- Placez le solvant (eau) dans une fiole Erlenmeyer distincte et ajoutez puces bouillante ou un bar de remuer à maintenir en douceur. Chauffer à ébullition sur une plaque chauffante.
- Ajouter solvant chaude dans un ballon à la température ambiante contenant le composé en petites portions, en agitant après chaque addition, jusqu'à ce que le composé soit complètement dissout.
- Pendant le processus de dissolution, conserver la solution chaude à tout moment en se reposant sur la plaque de cuisson, trop. N’ajoutez pas plus chaud solvant que nécessaire - juste assez pour dissoudre l’échantillon.
- Si une partie de l’état solide ne semble pas se dissoudre, même après que plus de solvant chaud a été ajouté, il est probable en raison de la présence d’impuretés très insolubles. Si cela se produit, arrêter l’ajout de solvant et faire une filtration à chaud avant de continuer.
- Pour effectuer une filtration à chaud, plier un morceau de papier filtre en forme de cône cannelé et placez-le dans un entonnoir de verre sans tige.
- Ajouter un excès de 10 à 20 % de solvant chaud à la solution d’eau chaude pour permettre l’évaporation dans la procédure.
- Verser la solution à travers le papier. Si les cristaux commence à se former à tout moment au cours du processus, ajoutez une petite partie du solvant chaude pour dissoudre.
3. la Solution de refroidissement
- Mettre la fiole contenant le composé dissous sur une surface qui ne réalise pas d’évacuer la chaleur trop rapidement, comme une serviette en papier sur une table de travail.
- Recouvrir légèrement le ballon en refroidissant pour éviter une évaporation et pour empêcher la poussière de tomber dans la solution.
- Laisser le flacon jusqu'à ce qu’il se refroidit jusqu'à la température ambiante.
- Une fois les cristaux ont formé, placer la solution dans un bain de glace pour s’assurer que le montant maximum de cristaux. Les solutions doivent être laissées intactes dans le bain de glace pendant 30 min à 1 h, ou jusqu'à ce que le composé semble avoir cristallisé complètement hors de la solution.
- Si aucune formation de cristaux n’est évidente, il peut être induite en grattant l’intérieur des murs de la fiole avec une baguette en verre ou en ajoutant un cristal de petite graine du même composé.
- Si cela ne fonctionne toujours pas, alors trop solvant a probablement été utilisé. Réchauffer la solution, permettre à certains du solvant à ébullition au large, puis refroidir.
4. isoler et sécher les cristaux
- Mettre la fiole froide contenant les cristaux néoformés sur une paillasse.
- Recouvrir légèrement le ballon pour éviter l’évaporation et pour empêcher la poussière de tomber dans la solution.
- Isoler les cristaux de filtration sous vide, avec entonnoir Büchner ou Hirsch (collier et une bague du ballon se tenir tout d’abord).
- Rincer les cristaux sur l’entonnoir Büchner avec une petite quantité de solvant fraîche et froide (le même solvant utilisé pour la recristallisation) pour éliminer toutes les impuretés qui peuvent s’en tenir aux cristaux.
- Pour sécher les cristaux, les laisser dans l’entonnoir filtrant et aspirer l’air à travers eux pendant plusieurs minutes. Cristaux peut également être séchés à l’air en leur permettant de rester non couvert pendant plusieurs heures ou jours. Des méthodes plus efficaces comprennent le séchage sous vide ou en plaçant dans un dessicateur.
| Solvant polaire | Moins solvant polaire |
| Acétate d’éthyle | Hexane |
| Méthanol | Chlorure de méthylène |
| Eau | Éthanol |
| Toluène | Hexane |
Le tableau 1. Paires de solvants communs.
Résultats
Un exemple des résultats de recristallisation est illustré à la Figure 2. Les jaunes impuretés présentes dans l’enceinte de brut ont été retirées, et le produit pur est laissé comme un solide blanc cassé. La pureté du composé recristallisé peut maintenant être vérifiée par spectroscopie de résonance magnétique nucléaire (RMN) ou, si c’est un composé avec un point de fusion publié, par la similitude son point de fusion est pour le point de fusion de la littérature. Si nécessaire, plusieurs Me10Si6 peut être effectuée jusqu'à ce que la pureté est suffisamment élevée.

La figure 2. 2 a) un brut composé 2 (à gauche), en b) recristallise le produit avant la filtration (au milieu) et 2 c) le même composé après recristallisation (à droite).
Applications et Résumé
Recristallisation est une méthode de purification d’un composé en enlevant toutes les impuretés qui pourraient être mélangées avec elle. Il fonctionne mieux lorsque le composé est très soluble dans un solvant à chaud, mais très insoluble dans la version froide du même solvant. Le composé doit être solide à température ambiante. Recristallisation est souvent utilisée comme une étape de nettoyage, après d’autres méthodes (telles que la chromatographie sur colonne ou extraction) qui sont efficaces pour enlever de grandes quantités d’impuretés, mais qui ne déclenchent pas la pureté de la substance finale à un niveau suffisamment élevé.
Recristallisation est la seule technique qui peut produire des monocristaux absolument pur et parfaits d’un composé. Ces cristaux peut être utilisé pour l’analyse aux rayons x, qui est l’autorité ultime dans la détermination de la structure et la forme tridimensionnelle d’une molécule. Dans ces cas, la recristallisation est autorisée à avancer très lentement, au cours des semaines et des mois, pour permettre le cristallin à la forme sans l’inclusion de toutes les impuretés. Verrerie spéciale est nécessaire pour permettre le solvant s’évapore aussi lentement que possible durant cette période, ou pour permettre le solvant très lentement mélanger avec un autre solvant dans lequel le composé est insoluble (appelée addition antisolvent).
L’industrie pharmaceutique a également fait une utilisation intensive de recristallisation, puisque c’est un moyen de purification plus facilement mis à l’échelle de la chromatographie sur colonne. 3 l’importance de la recristallisation dans des applications industrielles a déclenché des éducateurs afin de souligner la recristallisation dans le curriculum de laboratoire. 4 par exemple, le médicament Stavudine, qui sert à réduire les effets du VIH, est généralement isolé par cristallisation. 5 souvent, molécules ont plusieurs structures cristallines différentes disponibles, il est donc nécessaire pour la recherche évaluer et comprendre quelle forme cristalline est isolé sous quelles conditions, tel que le refroidissement des taux, composition du solvant et ainsi de suite. Ces formes cristalline différente ont des propriétés biologiques différentes ou être absorbé par l’organisme à des taux différents.
Une utilisation plus courante de recristallisation est la fabrication de Candi. Candi faite en dissolvant le sucre dans l’eau chaude au point de saturation. Bâtons en bois sont placés dans la solution et la solution n’est autorisée à refroidir et de s’évaporer lentement. Après plusieurs jours, les gros cristaux de sucre ont augmenté partout dans les bâtons en bois.
References
- Mayo, D. W.; Pike, R. M.; Forbes, D. C., Microscale organic laboratory : with multistep and multiscale syntheses. 5th ed.; J. Wiley & Sons: Hoboken, NJ, p xxi, 681 p (2011).
- Armarego, W. L. F.; Chai, C. L. L., Purification of laboratory chemicals. 5th ed.; Butterworth-Heinemann: Amsterdam ; Boston; p xv, 609 p (2003).
- Ray, P. C.; Tummanapalli, J. M. C.; Gorantla, S. R., Process for the large scale production of Stavudine. Google Patents: (2011).
- Hightower, T. R.; Heeren, J. D., Using a Simulated Industrial Setting for the Development of an Improved Solvent System for the Recrystallization of Benzoic Acid: A Student-Centered Project. Journal of Chemical Education 83 (11), 1663 (2006).
- Rohani, S.; Horne, S.; Murthy, K., Control of Product Quality in Batch Crystallization of Pharmaceuticals and Fine Chemicals. Part 1: Design of the Crystallization Process and the Effect of Solvent. Organic Process Research & Development 9 (6), 858-872 (2005).
Divulgations
- Mayo, D. W.; Pike, R. M.; Forbes, D. C., Microscale organic laboratory : with multistep and multiscale syntheses. 5th ed.; J. Wiley & Sons: Hoboken, NJ, p xxi, 681 p (2011).
- Armarego, W. L. F.; Chai, C. L. L., Purification of laboratory chemicals. 5th ed.; Butterworth-Heinemann: Amsterdam ; Boston; p xv, 609 p (2003).
- Ray, P. C.; Tummanapalli, J. M. C.; Gorantla, S. R., Process for the large scale production of Stavudine. Google Patents: (2011).
- Hightower, T. R.; Heeren, J. D., Using a Simulated Industrial Setting for the Development of an Improved Solvent System for the Recrystallization of Benzoic Acid: A Student-Centered Project. Journal of Chemical Education 83 (11), 1663 (2006).
- Rohani, S.; Horne, S.; Murthy, K., Control of Product Quality in Batch Crystallization of Pharmaceuticals and Fine Chemicals. Part 1: Design of the Crystallization Process and the Effect of Solvent. Organic Process Research & Development 9 (6), 858-872 (2005).
Tags
Passer à...
Vidéos de cette collection:

Now Playing
Purification des composés par recristallisation
Organic Chemistry
706.9K Vues

Introduction à la catalyse
Organic Chemistry
34.2K Vues

Montage d'un chauffage à reflux
Organic Chemistry
166.9K Vues

Réaliser des réactions en dessous de la température ambiante
Organic Chemistry
70.4K Vues

Transfert de solvants via une rampe à vide (ligne Schlenk)
Organic Chemistry
41.5K Vues

Dégazage des liquides par la technique "cycle geler-pomper-dégeler"
Organic Chemistry
56.0K Vues

Préparation de réactifs anhydres et équipement
Organic Chemistry
79.2K Vues

Séparation des mélanges par précipitation
Organic Chemistry
157.4K Vues

Extraction solide-liquide
Organic Chemistry
237.5K Vues

Utilisation d'un évaporateur rotatif (ou Rotovap) pour éliminer un solvant
Organic Chemistry
212.7K Vues

Distillation fractionnée
Organic Chemistry
333.7K Vues

Préparation de cristaux pour analyse par diffraction des rayons X
Organic Chemistry
32.3K Vues

Performing 1D Thin Layer Chromatography
Organic Chemistry
289.0K Vues

Chromatographie sur colonne
Organic Chemistry
359.4K Vues

Spectroscopie à résonance magnétique nucléaire (RMN)
Organic Chemistry
247.4K Vues