Purifying Compounds by Recrystallization
Overview
Source: Laboratory of Dr. Jimmy Franco - Merrimack College
Recrystallization is a technique used to purify solid compounds.1 Solids tend to be more soluble in hot liquids than in cold liquids. During recrystallization, an impure solid compound is dissolved in a hot liquid until the solution is saturated, and then the liquid is allowed to cool.2 The compound should then form relatively pure crystals. Ideally, any impurities that are present will remain in the solution and will not be incorporated into the growing crystals (Figure 1). The crystals can then be removed from the solution by filtration. Not all of the compound is recoverable — some will remain in the solution and will be lost.
Recrystallization is not generally thought of as a separation technique; rather, it is a purification technique in which a small amount of an impurity is removed from a compound. However, if the solubility properties of two compounds are sufficiently different, recrystallization can be used to separate them, even if they are present in nearly equal amounts. Recrystallization works best when most impurities have already been removed by another method, such as extraction or column chromatography.
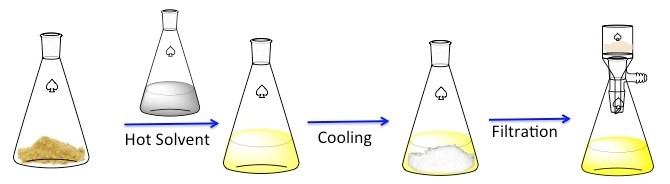
Figure 1. The general scheme for recrystallization.
Principles
A successful recrystallization depends on the proper choice of solvent. The compound must be soluble in the hot solvent and insoluble in the same solvent when it is cold. For the purpose of recrystallization, consider 3% w/v the dividing line between soluble and insoluble: if 3 g of a compound dissolves in 100 mL of a solvent, it is considered soluble. In choosing a solvent, the bigger the difference between hot solubility and cold solubility, the more product recoverable from recrystallization.
The rate of cooling determines the size and quality of the crystals: rapid cooling favors small crystals, and slow cooling favors the growth of large and generally purer crystals. The rate of recrystallization is usually greatest at about 50 °C below the melting point of the substance; the maximum formation of crystals occurs at about 100 °C below the melting point.
Although the terms "crystallization" and "recrystallization" are sometimes used interchangeably, they technically refer to different processes. Crystallization refers to the formation of a new, insoluble product by a chemical reaction; this product then precipitates out of the reaction solution as an amorphous solid containing many trapped impurities. Recrystallization does not involve a chemical reaction; the crude product is simply dissolved into solution, and then the conditions are changed to allow crystals to re-form. Recrystallization produces a more pure final product. For this reason, experimental procedures that produce a solid product by crystallization normally include a final recrystallization step to give the pure compound.
Procedure
Perform all steps in a fume hood to prevent exposure to solvent fumes.
1. Selecting a Solvent
- Place 50 mg of the sample (N-bromosuccinimide) in an Erlenmeyer flask.
- Add 0.5 mL of boiling solvent (water). If the sample dissolves completely, the solubility in the cold solvent is too high to be a good recrystallization solvent.
- If the sample does not dissolve in the cold solvent, heat the test tube until the solvent boils.
- If the sample has not completely dissolved at this point, add more boiling solvent drop-wise, until all of the solid dissolves. If it takes more than 3 mL to dissolve the sample in the hot solvent, the solubility in this solvent is probably too low to make it a good recrystallization solvent.
- If the first choice of solvent is not a good recrystallization solvent, try others. If a single solvent that works cannot be found, try a two solvent system.
- If you cannot find a suitable single solvent system, then a solvent pair may be necessary. When identifying a solvent pair, there are several key considerations 1) The first solvent should readily dissolve the solid. 2) The second solvent must be miscible with the 1st solvent, but have a much lower solubility for the solute.
- As a general rule "likes dissolve likes" meaning that polar compounds tend to be soluble in polar solvents and non-polar compounds are often more soluble non-polar compounds.
- Common solvent pairs (Table 1)
- Make sure the solvent has a boiling point of at least 40 °C, so there is a reasonable temperature difference between boiling solvent and room-temperature solvent.
- Ensure that the solvent has a boiling point below about 120 °C, so it's easier to remove the last traces of solvent from the crystals.
- Also make sure the boiling point of the solvent is lower than the melting point of the compound, so the compound forms as solid crystals rather than as an insoluble oil.
- Confirm that the impurities are either insoluble in the hot solvent (so they can be hot-filtered out, once the compound is dissolved) or soluble in the cold solvent (so they stay dissolved during the entire process).
2. Dissolving the Sample in Hot Solvent
- Place the compound to be recrystallized in an Erlenmeyer flask. This is a better choice than a beaker, since the sloping sides help trap solvent vapors and slow the rate of evaporation.
- Place the solvent (water) in a separate Erlenmeyer flask, and add boiling chips or a stir bar to keep it boiling smoothly. Heat it to boiling on a hotplate.
- Add hot solvent to a flask at room temperature containing the compound in small portions, swirling after each addition, until the compound is completely dissolved.
- During the dissolution process, keep the solution hot at all times by resting it on the hotplate, too. Do not add more hot solvent than necessary - just enough to dissolve the sample.
- If a portion of the solid does not seem to dissolve, even after more hot solvent has been added, it is likely due to the presence of very insoluble impurities. If this happens, stop adding solvent and do a hot filtration before proceeding.
- To perform a hot filtration, fold a piece of filter paper into a fluted cone shape and place it into a glass stemless funnel.
- Add a 10-20% excess of hot solvent to the hot solution to allow for evaporation in the procedure.
- Pour the solution through the paper. If crystals begin to form at any time during the process, add a small portion of warm solvent to dissolve them.
3. Cooling the Solution
- Set the flask containing the dissolved compound on a surface that does not conduct the heat away too quickly, such as a paper towel set on a benchtop.
- Lightly cover the flask as it cools to prevent evaporation and to prevent dust from falling into the solution.
- Leave the flask undisturbed until it cools to room temperature.
- Once the crystals have formed, place the solution in an ice bath to ensure that the maximum amount of crystals is obtained. The solutions should be left undisturbed in the ice bath for 30 min to 1 h, or till the compound appears to have completely crystalized out of solution.
- If no crystal formation is evident, it can be induced by scratching the inside walls of the flask with a glass rod or by adding a small seed crystal of the same compound.
- If this still fails to work, then too much solvent was probably used. Reheat the solution, allow some of the solvent to boil off, then cool it.
4. Isolating and Drying the Crystals
- Set the cold flask containing the newly formed crystals on a benchtop.
- Lightly cover the flask to prevent evaporation and to prevent dust from falling into the solution.
- Isolate the crystals by vacuum filtration, using either a Büchner or Hirsch funnel (clamp the flask to a ring stand first).
- Rinse the crystals on the Büchner funnel with a small amount of fresh, cold solvent (the same solvent used for recrystallization) to remove any impurities that may be sticking to the crystals.
- To dry the crystals, leave them in the filter funnel and draw air through them for several minutes. Crystals can also be air-dried by allowing them to stand uncovered for several hours or days. More efficient methods include vacuum drying or placing in a desiccator.
| Polar Solvent | Less Polar Solvent |
| Ethyl acetate | Hexane |
| Methanol | Methylene chloride |
| Water | Ethanol |
| Toluene | Hexane |
Table 1. Common solvent pairs.
Results
An example of the results of recrystallization is shown in Figure 2. The yellow impurities present in the crude compound have been removed, and the pure product is left as an off-white solid. The purity of the recrystallized compound can now be verified by nuclear magnetic resonance (NMR) spectroscopy or, if it is a compound with a published melting point, by how similar its melting point is to the literature melting point. If necessary, multiple recrystallizations can be performed until the purity is acceptably high.

Figure 2. 2a) A crude compound (left), 2b) recrystallized product before filtration (middle), and 2c) the same compound after recrystallization (right).
Application and Summary
Recrystallization is a method of purifying a compound by removing any impurities that might be mixed with it. It works best when the compound is very soluble in a hot solvent, but very insoluble in the cold version of the same solvent. The compound must be a solid at room temperature. Recrystallization is often used as a final clean-up step, after other methods (such as extraction or column chromatography) that are effective at removing larger amounts of impurities, but that do not raise the purity of the final compound to a sufficiently high level.
Recrystallization is the only technique that can produce absolutely pure, perfect single crystals of a compound. These crystals can be used for X-ray analysis, which is the ultimate authority in determining the structure and three-dimensional shape of a molecule. In these cases, the recrystallization is allowed to proceed very slowly, over the course of weeks to months, to allow the crystal lattice to form without the inclusion of any impurities. Special glassware is needed to allow the solvent to evaporate as slowly as possible during this time, or to allow the solvent to very slowly mix with another solvent in which the compound is insoluble (called antisolvent addition).
The pharmaceutical industry also makes heavy use of recrystallization, since it is a means of purification more easily scaled up than column chromatography.3 The importance of recrystallization in industrial applications has triggered educators to emphasize recrystallization in the laboratory curriculum.4 For example, the drug Stavudine, which is used to reduce the effects of HIV, is typically isolated by crystallization.5 Often, molecules have multiple different crystal structures available, so it is necessary for research to evaluate and understand which crystal form is isolated under what conditions, such as cooling rate, solvent composition, and so forth. These different crystal forms might have different biological properties or be absorbed into the body at different rates.
A more common use of recrystallization is in making rock candy. Rock candy is made by dissolving sugar in hot water to the point of saturation. Wooden sticks are placed into the solution and the solution is allowed to cool and evaporate slowly. After several days, large crystals of sugar have grown all over the wooden sticks.
References
- Mayo, D. W.; Pike, R. M.; Forbes, D. C., Microscale organic laboratory : with multistep and multiscale syntheses. 5th ed.; J. Wiley & Sons: Hoboken, NJ, p xxi, 681 p (2011).
- Armarego, W. L. F.; Chai, C. L. L., Purification of laboratory chemicals. 5th ed.; Butterworth-Heinemann: Amsterdam ; Boston; p xv, 609 p (2003).
- Ray, P. C.; Tummanapalli, J. M. C.; Gorantla, S. R., Process for the large scale production of Stavudine. Google Patents: (2011).
- Hightower, T. R.; Heeren, J. D., Using a Simulated Industrial Setting for the Development of an Improved Solvent System for the Recrystallization of Benzoic Acid: A Student-Centered Project. Journal of Chemical Education 83 (11), 1663 (2006).
- Rohani, S.; Horne, S.; Murthy, K., Control of Product Quality in Batch Crystallization of Pharmaceuticals and Fine Chemicals. Part 1: Design of the Crystallization Process and the Effect of Solvent. Organic Process Research & Development 9 (6), 858-872 (2005).
Disclosures
- Mayo, D. W.; Pike, R. M.; Forbes, D. C., Microscale organic laboratory : with multistep and multiscale syntheses. 5th ed.; J. Wiley & Sons: Hoboken, NJ, p xxi, 681 p (2011).
- Armarego, W. L. F.; Chai, C. L. L., Purification of laboratory chemicals. 5th ed.; Butterworth-Heinemann: Amsterdam ; Boston; p xv, 609 p (2003).
- Ray, P. C.; Tummanapalli, J. M. C.; Gorantla, S. R., Process for the large scale production of Stavudine. Google Patents: (2011).
- Hightower, T. R.; Heeren, J. D., Using a Simulated Industrial Setting for the Development of an Improved Solvent System for the Recrystallization of Benzoic Acid: A Student-Centered Project. Journal of Chemical Education 83 (11), 1663 (2006).
- Rohani, S.; Horne, S.; Murthy, K., Control of Product Quality in Batch Crystallization of Pharmaceuticals and Fine Chemicals. Part 1: Design of the Crystallization Process and the Effect of Solvent. Organic Process Research & Development 9 (6), 858-872 (2005).
Tags
Skip to...
Videos from this collection:

Now Playing
Purifying Compounds by Recrystallization
Organic Chemistry
707.3K Views

Introduction to Catalysis
Organic Chemistry
34.3K Views

Assembly of a Reflux System for Heated Chemical Reactions
Organic Chemistry
167.1K Views

Conducting Reactions Below Room Temperature
Organic Chemistry
70.4K Views

Schlenk Lines Transfer of Solvents
Organic Chemistry
41.6K Views

Degassing Liquids with Freeze-Pump-Thaw Cycling
Organic Chemistry
56.0K Views

Preparing Anhydrous Reagents and Equipment
Organic Chemistry
79.3K Views

Separation of Mixtures via Precipitation
Organic Chemistry
157.5K Views

Solid-Liquid Extraction
Organic Chemistry
237.6K Views

Rotary Evaporation to Remove Solvent
Organic Chemistry
212.7K Views

Fractional Distillation
Organic Chemistry
333.9K Views

Growing Crystals for X-ray Diffraction Analysis
Organic Chemistry
32.3K Views

Performing 1D Thin Layer Chromatography
Organic Chemistry
289.1K Views

Column Chromatography
Organic Chemistry
359.6K Views

Nuclear Magnetic Resonance (NMR) Spectroscopy
Organic Chemistry
247.4K Views
Copyright © 2025 MyJoVE Corporation. All rights reserved