重结晶法净化化合物
Overview
资料来源: 实验室的博士吉米 · 佛朗哥-梅里马克大学
再结晶是一种用于净化固体化合物技术。1固体倾向于更溶于热的液体比冷液体中。在再结晶,不纯的固体化合物溶解在热的液体直到饱和溶液,然后允许的液体冷却。2该化合物应该然后形成相对纯净的晶体。理想情况下,存在任何杂质始终在解决方案中,将不会合并成生长晶体 (图 1)。晶体,可以用过滤法去除从解决方案。不是所有的这种化合物是可采 — — 部分将留在解决方案中,而且将会丢失。
再结晶是不普遍认为是一种分离技术;相反,它是极少量的杂质从一种化合物的纯化技术。然而,如果两种化合物的溶解性能足够不同,再结晶可用于将它们分开,即使他们目前在几乎相等的金额。时的大部分杂质已被删除的其他方法,如提取或柱层析,再结晶的效果最好。
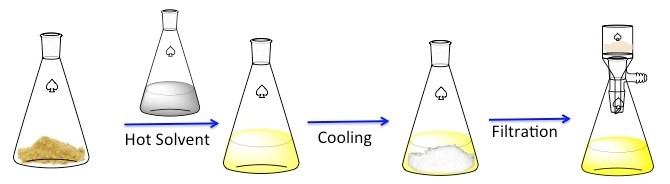
图 1。再结晶的一般方案。
Principles
成功的再结晶取决于溶剂的正确选择。冷的时候,必须溶于热溶剂,不溶于同一溶剂化合物。再结晶,否 3 %w / v 的分界线之间可溶性和不溶性: 如果 3g 的一种化合物溶解在 100 毫升的一种溶剂,它被认为是可溶性。在选择从再结晶溶剂,热溶解度和冷溶解度之间的差异越大,可恢复更多的产品。
冷却速率确定大小和晶体质量: 快速冷却有利于小水晶,和缓慢冷却有利于大和一般纯净的晶体的生长。再结晶速度通常最伟大的约 50 ° C 低于熔点的物质;晶体的最大形成发生在约 100 ° C 以下的熔点。
虽然有时交替使用的术语"结晶"和"结晶",他们从技术上讲是指不同的过程。结晶是指形成新的、 不溶性的产品的一种化学反应;本产品然后沉淀反应解决方案作为包含很多被困的杂质无定形固体。再结晶并不涉及化学反应;粗品简单地溶解,然后改变条件允许,重新形成的晶体。再结晶产生更纯的最终产物。为此,通常采用结晶法生产固体产物的实验程序包括一个最后再结晶步骤给纯化合物。
Procedure
在油烟罩,以防止接触溶剂烟雾中执行所有步骤。
1.选择一种溶剂
- 在锥形瓶中放置 50 毫克的样品 (N-丁二)。
- 添加 0.5 毫升的沸点溶剂 (水)。如果样品完全溶解,在冷溶剂中的溶解度是太高,不好再结晶溶剂。
- 如果样品不溶于冷溶剂,加热试管,直到溶剂沸腾。
- 如果样品已不完全地被溶化在这一点上,落,添加更多的沸点溶剂,直到所有的固体溶解。如果需要超过 3 毫升溶解热溶剂中的示例,在该溶剂中的溶解度是可能太低,无法使它好重结晶溶剂。
- 如果第一溶剂选择的不是一种好的重结晶溶剂,再试试其它。如果找不到工作的单一溶剂,尝试两种溶剂系统。
- 如果你找不到合适的单一溶剂体系,溶剂对可能有必要。在确定时溶剂的双,有几个关键注意事项 1) 第一溶剂应迅速溶解固体。2) 第二溶剂必须与 1st溶剂混溶,但有多低水中溶解度的溶质。
- 作为一般规则"喜欢溶解喜欢"意思,极性化合物往往是溶于极性溶剂和非极性化合物往往更多可溶性非极性化合物。
- 常见的双溶剂 (表 1)
- 请确保溶剂有至少 40 ° C,沸点,因此沸腾溶剂和房间温度溶剂的合理温差。
- 确保溶剂沸点低于约 120 ° C,所以很容易从晶体中删除溶剂的最后痕迹。
- 此外请确保溶剂的沸点是低于熔点的化合物,所以这种化合物形成固态晶体,而不是一种不溶性的油。
- 确认的杂质是要么不溶于热溶剂 (这样他们可以热过滤掉,一旦溶解的化合物) 或溶于冷溶剂 (所以在整个过程中,他们留下来溶解)。
2.溶解热溶剂中的示例
- 这种化合物的地方进行再结晶在锥形瓶中。这是一个更好的选择,比一只量杯,由于倾斜的侧面帮助陷阱溶剂蒸气和蒸发的速度放慢。
- 将溶剂 (水) 放在单独的锥形瓶,并添加沸腾芯片或搅拌棒,让它顺利地沸腾。加热到沸腾的电炉子上。
- 将热溶剂添加到包含该化合物在小部分,旋转后再加,直到完全溶解的化合物的室温一瓶。
- 在溶解过程中,保持溶液热在任何时候都通过它置于烤盘,太。不添加更多不必要的热溶剂溶解样品只够。
- 如果固体部分似乎没有溶解,甚至增加了更多的热溶剂后,它可能是因为非常不溶性杂质的存在。如果发生这种情况,停止添加溶剂和之前的热过滤。
- 执行热过滤、 折叠一张过滤纸,成槽的锥形状并将它放入玻璃无茎漏斗。
- 10-20%过量的热溶剂向解决方案中添加热,以便在过程中的蒸发。
- 倒通过本文解决方案。如果晶体开始形成过程中的任何时候,添加温暖的溶剂来溶解他们一小部分。
3.冷却解决方案
- 设置瓶含有溶解的化合物,在不进行热走得太快,如在台式上设置一张纸巾的表面。
- 当它冷却时防止蒸发,防止灰尘落入解决方案,轻轻披烧瓶。
- 离开烧瓶原状,直到它冷却到室温。
- 一旦形成了晶体,将解决方案在冰浴,以确保得到晶体的最大量。解决方案应由原状在冰浴 30 分钟到 1 小时,或者直到这种化合物似乎已经完全从溶液结晶。
- 如果没有晶体的形成是显而易见的它能诱导抓里面的烧瓶用玻璃棒或通过添加同一化合物小种子水晶墙。
- 如果这仍然不能工作,那么很可能用太多的溶剂。再热解决方案,允许一些溶剂来烧掉,然后冷却。
4.孤立和干燥晶体
- 设置包含台式新形成的晶体的冷瓶。
- 轻轻地盖瓶防止蒸发,防止灰尘落入解决方案。
- 通过真空过滤,使用 Büchner 或美国好施集团漏斗 (夹紧环瓶第一站) 隔离的晶体。
- 冲洗用少量的新鲜、 冷溶剂 (相同的溶剂,用于再结晶) 布氏漏斗上的晶体来删除任何可能坚持晶体的杂质。
- 干晶体,把它们留在过滤漏斗和空气通过他们绘制了几分钟。也可以通过允许他们站发现了几个小时或天风干晶体。更有效的方法包括真空干燥或置于干燥器。
| 极性溶剂 | 低极性的溶剂 |
| 乙酸乙酯 | 正己烷 |
| 甲醇 | 二氯甲烷 |
| 水 | 乙醇 |
| 甲苯 | 正己烷 |
表 1。常见的双溶剂。
Results
再结晶的结果示例如图 2所示。黄色的杂质存在于原油的化合物已被删除,和作为一种白色的固体留下的纯的产品。再结晶化合物纯度现在可通过核磁共振 (NMR) 来验证,或如果它是与已发布的熔点的化合物,由如何类似它的熔点是文学熔点。如有必要,可以执行多个重,直到纯度是可接受的高。

图 2。2a) 原油的复合 (左)、 2b) 重结晶产品过滤 (中间) 前, 和 2 c) 相同复合后再结晶 (右图)。
Application and Summary
再结晶是提纯化合物通过删除任何可能混入的杂质的方法。这种化合物是非常溶于热的溶剂,但不是溶于水的相同溶剂的冷版本效果最好。这种化合物必须在室温下是固体。再结晶通常用作清理的最后一步之后其他方法 (如萃取或柱色谱法),可以有效的去除大量的杂质,但那不提高纯度的最后化合物对一个足够高的水平。
再结晶是唯一可以产生一种化合物的绝对纯净,完美晶体的技术。这些晶体可以用于 x 射线衍射分析,是在确定的结构和一种分子的三维形状的终极权威。在这些情况下,再结晶被允许进行得非常缓慢,个星期到几个月,允许晶格形成不含任何杂质的夹杂,当然。特殊的玻璃器皿被需要让溶剂蒸发尽可能慢地在这段时间,或允许非常缓慢混合,这种化合物是不溶性 (称为抗溶剂添加) 的另一种溶剂的溶剂。
制药业也利用重结晶,因为它是易放大比柱层析纯化更多的手段。3在工业应用中的再结晶的重要性引发了教育工作者要强调实验室课程中的再结晶。4为例,药物司他夫定,用来减少艾滋病毒的影响,通常采用结晶法分离。5通常情况下,分子有多个不同的晶体结构可用,因此有必要研究来评估和了解晶体形成孤立根据什么条件,如冷却率、 溶剂组成,等等。这些不同晶型可能有不同的生物学特性,或被吸收到身体以不同的速率。
再结晶更常见用途是制作冰糖。冰糖是由溶解在热水里到饱和点糖。木棍被放入该解决方案,解决方案允许冷却和慢慢地蒸发。几天后,糖的大晶体有长满木棍。
References
- Mayo, D. W.; Pike, R. M.; Forbes, D. C., Microscale organic laboratory : with multistep and multiscale syntheses. 5th ed.; J. Wiley & Sons: Hoboken, NJ, p xxi, 681 p (2011).
- Armarego, W. L. F.; Chai, C. L. L., Purification of laboratory chemicals. 5th ed.; Butterworth-Heinemann: Amsterdam ; Boston; p xv, 609 p (2003).
- Ray, P. C.; Tummanapalli, J. M. C.; Gorantla, S. R., Process for the large scale production of Stavudine. Google Patents: (2011).
- Hightower, T. R.; Heeren, J. D., Using a Simulated Industrial Setting for the Development of an Improved Solvent System for the Recrystallization of Benzoic Acid: A Student-Centered Project. Journal of Chemical Education 83 (11), 1663 (2006).
- Rohani, S.; Horne, S.; Murthy, K., Control of Product Quality in Batch Crystallization of Pharmaceuticals and Fine Chemicals. Part 1: Design of the Crystallization Process and the Effect of Solvent. Organic Process Research & Development 9 (6), 858-872 (2005).
Disclosures
- Mayo, D. W.; Pike, R. M.; Forbes, D. C., Microscale organic laboratory : with multistep and multiscale syntheses. 5th ed.; J. Wiley & Sons: Hoboken, NJ, p xxi, 681 p (2011).
- Armarego, W. L. F.; Chai, C. L. L., Purification of laboratory chemicals. 5th ed.; Butterworth-Heinemann: Amsterdam ; Boston; p xv, 609 p (2003).
- Ray, P. C.; Tummanapalli, J. M. C.; Gorantla, S. R., Process for the large scale production of Stavudine. Google Patents: (2011).
- Hightower, T. R.; Heeren, J. D., Using a Simulated Industrial Setting for the Development of an Improved Solvent System for the Recrystallization of Benzoic Acid: A Student-Centered Project. Journal of Chemical Education 83 (11), 1663 (2006).
- Rohani, S.; Horne, S.; Murthy, K., Control of Product Quality in Batch Crystallization of Pharmaceuticals and Fine Chemicals. Part 1: Design of the Crystallization Process and the Effect of Solvent. Organic Process Research & Development 9 (6), 858-872 (2005).
Tags
跳至...
此集合中的视频:

Now Playing
重结晶法净化化合物
Organic Chemistry
707.6K Views

催化导论
Organic Chemistry
34.4K Views

程序集的激烈化学反应回流系统
Organic Chemistry
167.1K Views

进行室温下反应
Organic Chemistry
70.4K Views

溅镀转移的溶剂
Organic Chemistry
41.6K Views

随着冻融泵循环脱气液体
Organic Chemistry
56.1K Views

制备无水试剂和设备
Organic Chemistry
79.3K Views

通过沉淀混合物的分离
Organic Chemistry
157.6K Views

固-液萃取
Organic Chemistry
237.7K Views

旋转蒸发来去除溶剂
Organic Chemistry
212.8K Views

分馏
Organic Chemistry
334.0K Views

X 射线衍射分析晶体生长
Organic Chemistry
32.4K Views

Performing 1D Thin Layer Chromatography
Organic Chemistry
289.2K Views

柱层析法
Organic Chemistry
359.7K Views

核磁共振 (NMR)
Organic Chemistry
247.5K Views
版权所属 © 2025 MyJoVE 公司版权所有,本公司不涉及任何医疗业务和医疗服务。