Sintesi di un complesso di cobalto (II) legato ad ossigeno
Panoramica
Fonte: Deepika Das, Tamara M. Powers, Dipartimento di Chimica, Texas A & M University
La chimica bioinorganica è il campo di studio che indaga il ruolo che i metalli svolgono in biologia. Circa la metà di tutte le proteine contiene metalli e si stima che fino a un terzo di tutte le proteine si basi su siti attivi contenenti metalli per funzionare. Le proteine che presentano metalli, chiamate metalloproteine, svolgono un ruolo vitale in una varietà di funzioni cellulari necessarie per la vita. Le metalloproteine hanno incuriosito e ispirato i chimici inorganici sintetici per decenni e molti gruppi di ricerca hanno dedicato i loro programmi alla modellazione della chimica dei siti attivi contenenti metalli nelle proteine attraverso lo studio dei composti di coordinazione.
Il trasporto di O2 è un processo vitale per gli organismi viventi. O2-metalloproteine di trasporto sono responsabili del legame, del trasporto e del rilascio di ossigeno, che può quindi essere utilizzato per processi vitali come la respirazione. Il complesso di coordinazione del cobalto che trasporta ossigeno, [N,N'-bis (salicilaldeide) etilendiaimino] cobalto (II) [Co (salen)]2 è stato ampiamente studiato per comprendere come i complessi metallici legano reversibilmente O2. 1
In questo esperimento, sintetizzaremo [Co(salen)]2 e studieremo la sua reazione reversibile con O2 in presenza di dimetilsolfossido (DMSO). In primo luogo, quantificheremo la quantità di O2 consumata all'esposizione di [Co(salen)]2 a DMSO. Osserveremo quindi visivamente il rilascio di O2 dall'addotto [Co(salen)]2-O2 esponendo il solido a CHCl3.
Principi
Esistono due polimorfi solidi di [Co(salen)]2 (attivo e inattivo), che possono essere isolati da diverse condizioni di reazione. Attivo e inattivo [Co(salen)]2 variano nel loro colore (marrone e rosso, rispettivamente), struttura e reattività. Entrambi i polimorfi sono costituiti da unità dimeriche. Nel caso di [Co(salen)]attivo 2, i Co-centri in ciascuna delle due molecole di Co(salen)2 sono in stretta vicinanza, formando un'interazione di van der Waals molto debole tra i centri metallici (Figura 1). Mentre la forma attiva mostra una debole interazione Co-Co, la separazione tra le unità dimeriche fornisce spazio per O2 per reagire con i centri Co; di conseguenza, la forma attiva di [Co(salen)]2 reagisce con O2 allo stato solido.
Nella cosiddetta forma inattiva di [Co(salen)]2, esiste un'interazione dativa tra il centro Co di una molecola e un atomo di ossigeno dall'altra (Figura 1). Le due unità Co(salen)2 sono più vicine tra loro rispetto alla forma attiva e, di conseguenza, la forma inattiva è stabile nell'aria allo stato solido e reagisce con O2 solo in presenza di un solvente coordinante (come DMSO), che interrompe l'unità dimerica e stabilizza l'addotto [Co(salen)]2-O2. Inattivo [Co(salen)]2 è più facile da maneggiare e studiare, poiché il solido può essere isolato senza utilizzare tecniche prive di aria. Pertanto, in questo esperimento sintetizzaremo [Co(salen)]2 inattivo e studieremo la sua reazione con O2 in presenza di DMSO.
Esistono diversi modi in cui O2,una molecola biatomica, può coordinarsi con i centri metallici (Figura 2). Il legame finale si traduce in un legame metallo-ossigeno a uno degli atomi di ossigeno in O2. Nel legame laterale, entrambi gli atomi di ossigeno formano legami con il centro metallico. In alcuni casi, l'unità O2 collega due complessi metallici in cui si osserva anche la legatura end-on e side-on.
Inattivo [Co(salen)]2 forma un prodotto da cobalto 2:1 a O2 in presenza del solvente di coordinamento, DMSO. L'unità O2 collega i due centri di cobalto in modo ponte finale(Figura 3)e le molecole DMSO coordinate completano la sfera di coordinazione ottaedrica di ciascuno dei centri Co. Se consideriamo il diagramma MO di O2 e d-diagramma di scissione orbitale per [Co(salen)]2, possiamo capire perché l'addotto 2:1 O2 è favorito (Figura 4). O2 mostra uno stato base tripletta con due elettroni spaiati nel π* MO. [Co(salen)]2 è paramagnetico, con un elettrone spaiato nella sua σ*dz2 MO (assumendo planare quadrato (D4h), Co2+, 7 de-). Il legame di O2 a [Co(salen)]2 è una reazione redox, in cui due molecole di Co(salen) sono ossidate da 1 e- ciascuna ad uno stato di ossidazione finale di +3 al cobalto, e la molecola di O2 è ridotta di 2 e-,con conseguente formazione di perossido (O22-). L'addotto 1:1 non è favorito in questo caso perché Co(III) è d6 e, quindi, non vuole rinunciare ad un altro elettrone (Per una recensione sulla teoria MO/d-scissione orbitale, vedi il video su Teoria dei Gruppi e Teoria MO dei Complessi di Metalli di Transizione).
In questo video, determineremo sperimentalmente il rapporto Co:O2 in caso di reazione di [Co(salen)]inattivo con O2 in presenza di DMSO misurando il volume di O2 perso in un sistema chiuso. Possiamo usare la legge del gas ideale (Equazione 1) per calcolare il numero di moli di O2 consumati.
PV = nRT (Equazione 1)
P = pressione = 1 atm
V = volume (L)
R = 0,082 L atm mol-1 K-1
T = temperatura (K)
n = talpe
Studieremo quindi la reversibilità del legame O2 esponendo il solido risultante [Co(salen)]2-O2-(DMSO)2 al cloroformio (CHCl3). L'aggiunta di CHCl3 (un solvente non coordinante che non può stabilizzare l'addotto [Co(salen)]2-O2) porta ad una diminuzione della concentrazione di DMSO. Il principio di Le Châtelier può spiegare che in caso di diminuzione della concentrazione di DMSO, l'equilibrio mostrato in Figura 3 si sposterà verso i reagenti, con conseguente liberazione del gas O2.
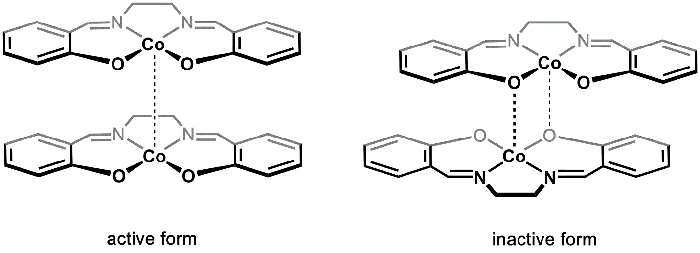
Figura 1. Forme attive e inattive di [Co(salen)]2.
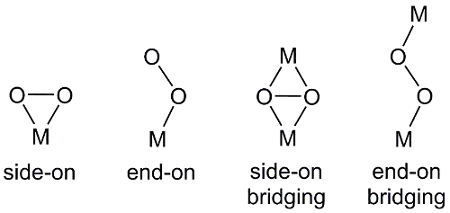
Figura 2. Modalità di coordinamento di O2 al centro metallico, M.

Figura 3. Reazione reversibile di O2 con [Co(salen)]2.
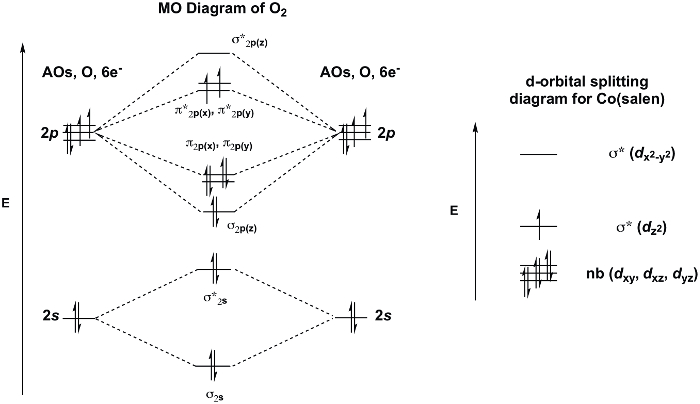
Figura 4. Diagramma MO di O2 e d-diagramma di scissione orbitale di Co(salen) (derivato dalla teoria dei gruppi, assumendo geometria planare quadrata).
Procedura
1. Sintesi di Inattivo [Co(salen)]2
- Caricare un pallone rotondo a fondo tondo a 3 colli da 250 mL con 120 mL di EtOH al 95% e 2,20 g (0,192 mL, 0,018 mol) di salicilaldeide.
- Montare il collo centrale con un condensatore collegato a N2. Montare gli altri due colli con un setto di gomma e un imbuto aggiuntivo dotato di un setto di gomma.
- Mescolare la reazione a bagnomaria e riscaldare la soluzione a reflusso (80 °C).
- Aggiungere etilene diammina (0,52 g, 0,58 ml, 0,0087 mol) attraverso la siringa attraverso il setto del matraccio a fondo tondo.
- In un matraccio a fondo tondo da 50 mL, preparare una soluzione di Co(OAc)2·4H 2O (2,17 g, 0,0087 mol) in 15 mL di acqua distillata. Riscaldare la soluzione nello stesso bagno d'acqua contenente il matraccio a 3 colli per assicurarsi che tutto l'acetato di cobalto si dissolva.
- Aggiungere la soluzione di acetato di cobalto all'imbuto di aggiunta.
- Degassare la soluzione di acetato di cobalto facendo gorgogliare N2 attraverso il liquido nell'imbuto di addizione per 10 minuti (vedere il video "Sintesi di un metallocene Ti(III) usando la tecnica della linea schlenk" per una procedura più dettagliata sullo spurgo dei liquidi). Potrebbe essere necessario chiudere l'adattatore N2 del condensatore per consentire all'N2 di bollire attraverso la soluzione di acetato di cobalto.
NOTA: Non riscaldare mai un sistema chiuso! Assicurarsi di sfiatare il sistema durante il degasaggio. - Aggiungere lentamente la soluzione di acetato di cobalto (II) (~ 1 goccia/s), mescolando vigorosamente la miscela di etanolo. Senza sufficiente agitazione, si formerà un precipitato grosso che può inceppare la barra di agitazione.
- Una volta aggiunto tutto l'acetato di cobalto, mescolare la reazione al reflusso per 1 ora.
- Spegnere la piastra calda e rimuovere il pallone a fondo tondo a 3 colli dal bagno d'acqua.
- Rimuovere il condensatore e l'imbuto di aggiunta dal pallone. Immergere il matraccio in un bagno di ghiaccio per facilitare la precipitazione del [Co(salen)]2.
- Filtrare la soluzione sotto vuoto per isolare il solido e lavare il solido rosso risultante con etanolo freddo.
- Isolate il solido. Calcola la resa della reazione e raccogli un IR del [Co(salen)]2. Assicurarsi che [Co(salen)]2 sia asciutto prima di utilizzarlo nella reazione di assorbimento di O2.
2. Configurazione dell'apparecchio per l'assorbimento di O2 (Figura 5)1
Nota: è molto importante che il sistema non perda. Una perdita nel sistema porterà a un rapporto Co:O2 inferiore al previsto.
- Collegare un ago a una bombola di gas O2 (ultra-elevata purezza) con tubo Tygon. Bolle delicatamente da O2 a 5 ml di DMSO per almeno 10 minuti.
- Mentre il DMSO è saturo di O2,montare le due estremità di una pipetta di vetro graduata da 10 ml con tubi Tygon (ciascuno di 1,5 piedi di lunghezza).
- Attaccare un imbuto di vetro a uno dei pezzi di tubi Tygon.
- Bloccare la pipetta di vetro e l'imbuto su un supporto ad anello in modo che l'imbuto sia rivolto verso l'alto e il tubo formi una forma a U ( Figura 5).
- Riempire la pipetta e l'imbuto con olio minerale. Aggiungere l'olio attraverso l'imbuto, assicurandosi che l'olio riempia anche il tubo collegato alla pipetta. Continuare ad aggiungere l'olio fino a quando l'imbuto non viene riempito a circa metà dell'imbuto. Non lasciare che l'olio si avvicini troppo alla parte superiore dell'imbuto, poiché l'O2 che gorgoglia attraverso l'imbuto può causare schizzi se l'imbuto è troppo pieno.
- All'estremità aperta del tubo, collegare una provetta a braccio laterale (provetta A).
- Aggiungere 50 mg (0,077 mmol) dell'inattivo [Co(salen)]2 alla provetta A del braccio laterale collegata alla pipetta di vetro.
- Aggiungere 2 mL di DMSO saturo di O2 a una provetta da 3 mL (provetta B).
- Utilizzare un paio di pinzette per abbassare delicatamente la provetta B nella provetta A, facendo attenzione a non versare alcun DMSO. È importante non esporre il [Co(salen)]2 al DMSO a questo punto.
- Sigillare la provetta A con un setto di gomma. Cablare il setto per evitare perdite.
- Inserire l'ago collegato al serbatoio del gas O2 nel setto e spurgare il sistema con O2 per 10 minuti.
- Rimuovere l'ago O2 e ungere la parte superiore del setto di gomma per evitare perdite.
- Potrebbe essere necessario rilasciare parte della pressione all'interno della configurazione per ottenere olio nella pipetta di vetro. Per fare ciò, inserire un ago libero nel setto di gomma sulla provetta A. Coprire l'apertura con un dito e rilasciare lentamente la pressione all'interno della configurazione. Non dimenticare di coprire il nuovo foro con grasso per evitare perdite.
- Spostare la pipetta di vetro e l'imbuto in modo che i livelli dell'olio si allineino in entrambi i pezzi di vetro.
- Registrare il livello di volume dell'olio all'interno della pipetta di vetro.

Figura 5. Configurazione dell'apparato di assorbimento O2.
3. O2 Reazione di assorbimento
- Aggiungere il DMSO al solido [Co(salen)]2 rovesciando delicatamente le provette, assicurandosi che nessuna delle soluzioni entri nel braccio laterale della provetta A.
- Una volta aggiunto tutto il DMSO, tenere premuta la parte superiore della provetta e mescolare delicatamente la soluzione agitando la provetta avanti e indietro.
NOTA: non utilizzare un movimento di agitazione su e giù. Sbattere le due provette insieme troppo violentemente può portare alla rottura della provetta A. - Continuare a scuotere delicatamente le provette a mano fino a quando il livello dell'olio nella pipetta smette di salire (circa 15-20 min).
- Una volta cessato il consumo di O2, spostare la pipetta e l'imbuto in modo che i livelli dell'olio si allineino.
- Registrare il nuovo livello di volume dell'olio nella pipetta di vetro. La differenza di volume è il volume di O2 consumato durante la reazione alla pressione atmosferica (1 atm).
- Registrare la temperatura della stanza.
4. O2 Liberazione da [Co(salen)]2 - O2 Addotto
- Trasferire la soluzione DMSO risultante dalla fase 3 a un tubo centrifugo da 15 mL.
- Riempire una seconda provetta con una quantità equivalente di acqua.
- Inserire le provette l'una di fronte all'altra in una centrifuga.
- Centrifugare il campione per almeno 15 min. La qualità del pellet solido risultante migliora con l'aumentare del tempo di centrifuga.
- Rimuovere delicatamente la provetta con il campione di addotto [Co(salen)]2-O2, in modo da non disturbare il pellet.
- Decantare accuratamente la soluzione DMSO sopra il pellet.
- Tenendo il tubo della centrifuga ad un angolo di 45 ° con il pellet rivolto verso l'alto, aggiungere lentamente 1 mL di CHCl3 con una pipetta, lasciando gocciolare la soluzione lungo il lato del tubo della centrifuga. Prestare estrema attenzione a non disturbare l'addotto solido [Co(salen)]2-O2.
- Osservare eventuali cambiamenti fisici che si verificano.
Risultati
Caratterizzazione di Inattivo [Co(salen)]2:
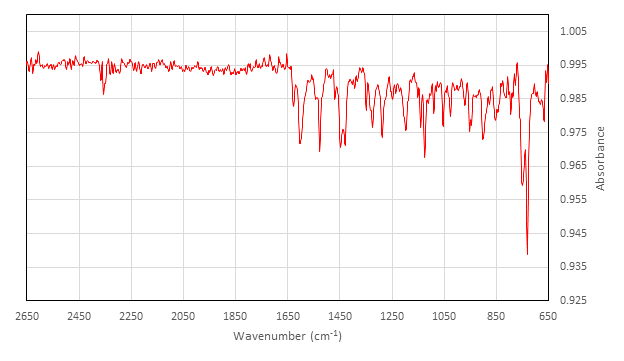
IR (cm-1) raccolti su attacco ATR: 2357 (w), 1626 (w), 1602 (m), 1542 (w), 1528 (m), 1454 (w), 1448 (m), 1429 (m), 1348 (w), 1327 (w), 1323 (m), 1288 (m), 1248 (w), 1236 (w), 1197 (m), 1140 (m), 1124 (m), 1089 (w), 1053 (m), 1026 (w), 970 (w), 952 (w), 947 (w), 902 (m), 878 (w), 845 (w), 813 (w), 794 (w), 750 (s), 730 (s).
O2 Assorbimento:
59,2 mg (0,090 mmol) di [Co(salen)]2 consumato 0,002 L di O2. Utilizzando la pressione standard e la temperatura registrata nel punto 3.6, il numero di moli di O2 consumati è stato:

Le moli calcolate di Co in 0,090 mmol di [Co(salen)]2:
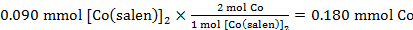
Pertanto il rapporto Co:O2 era:
0.180 mmol Co : 0.082 mmol O2
che equivale ad un rapporto 2:0.91 di Co a O2.
Aggiunta di CHCl3 a [Co(salen)]2–O2 Adduct:
Dopo l'aggiunta di CHCl3, la soluzione chCl3 è diventata rossa e un flusso di bolle è stato liberato dal solido, indicando il rilascio di gas O2 e la formazione di [Co(salen)]inattivo 2.
Applicazione e Riepilogo
In questo video, abbiamo spiegato i diversi modi in cui l'ossigeno biatomico può coordinarsi con i centri metallici. Abbiamo sintetizzato il complesso di cobalto che trasporta ossigeno [Co(salen)]2 e studiato il suo legame reversibile con O2. Sperimentalmente abbiamo dimostrato che [Co(salen)]2 inattivo lega reversibilmente O2 e forma un addotto 2:1 Co:O2 in presenza di DMSO.
Tutti i vertebrati dipendono dall'emoglobina, una metalloproteina presente nei globuli rossi, per trasportare l'ossigeno agli organi respiratori e ad altri tessuti. Nell'emoglobina, l'ossigeno si lega in modo reversibile a un gruppo eme che presenta un singolo centro Fe coordinato ad un anello eterociclico chiamato porfirina (Figura 6a). L'emoglobina non è l'unica metalloproteina che trasporta ossigeno e di stoccaggio. Ad esempio, i molluschi possiedono una proteina chiamata emocianina, che presenta un sito attivo del dirame responsabile del trasporto di ossigeno (Figura 6b).
L'uso di specie molecolari sintetiche per modellare siti attivi nelle metalloproteine è difficile a causa delle differenze distinte nella struttura elettronica di un semplice composto di coordinazione rispetto a quello di un metallo circondato da una sovrastruttura proteica. Di conseguenza, è spesso difficile replicare con precisione la struttura del sito attivo nelle metalloproteine. Mentre ci sono esempi di complessi di modelli che imitano strutturalmente i siti attivi dei metalli, ci sono meno esempi di complessi di modelli strutturalmente simili che mostrano reattività inerente al metalloenzima nativo.
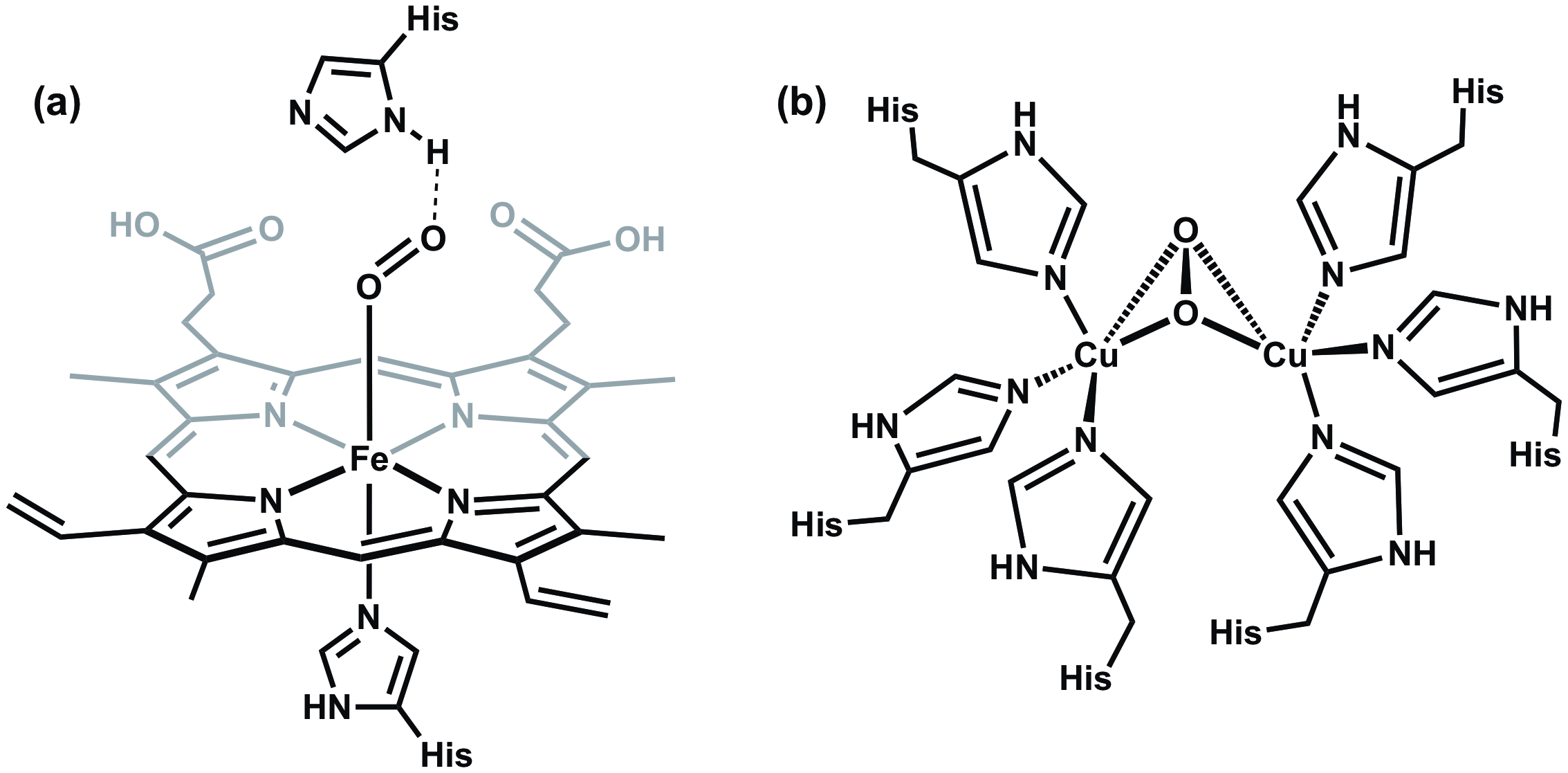
Figura 6. (a) Il centro Fe nell'emoglobina si lega a O2 in modo end-on, mentre (b) il rame contenente sito attivo nell'emocianina si lega a O2 in un orientamento ponte laterale.
Vai a...
Video da questa raccolta:

Now Playing
Sintesi di un complesso di cobalto (II) legato ad ossigeno
Inorganic Chemistry
51.8K Visualizzazioni

Sintesi di un Ti(III) metallocene utilizzando la tecnica della linea Schlenk
Inorganic Chemistry
31.6K Visualizzazioni

Scatola a guanti (Glove Box) e sensori di impurezze
Inorganic Chemistry
18.7K Visualizzazioni

Purificazione del ferrocene per sublimazione
Inorganic Chemistry
54.7K Visualizzazioni

Il metodo di Evans
Inorganic Chemistry
68.7K Visualizzazioni

Diffrazione a raggi X su cristallo singolo e su polveri
Inorganic Chemistry
105.1K Visualizzazioni

Spettroscopia di risonanza paramagnetica elettronica (EPR)
Inorganic Chemistry
25.6K Visualizzazioni

Spettroscopia Mössbauer
Inorganic Chemistry
22.0K Visualizzazioni

Interazione acido-base di Lewis in Ph3P-BH3
Inorganic Chemistry
39.0K Visualizzazioni

Struttura del ferrocene
Inorganic Chemistry
79.8K Visualizzazioni

Applicazione della teoria dei gruppi nella spettroscopia infrarossa
Inorganic Chemistry
45.9K Visualizzazioni

Teoria degli orbitali molecolari
Inorganic Chemistry
35.5K Visualizzazioni

Paddlewheel a quadruplo legame metallo-metallo
Inorganic Chemistry
15.3K Visualizzazioni

Celle di Grätzel (Dye-sensitized Solar Cells)
Inorganic Chemistry
16.0K Visualizzazioni

Inizio fotochimico di una reazione di polimerizzazione radicalica
Inorganic Chemistry
17.1K Visualizzazioni