Tecniche basate sull'immuno-precipitazione: purificazione di proteine endogene con l'impiego di microsfere di agarosio
Panoramica
Fonte: Susannah C. Shissler1, Tonya J. Webb1
1 Dipartimento di Microbiologia e Immunologia, Università del Maryland, Baltimora, MD 21201
L'immunoprecipitazione (IP, noto anche come test "pull-down") è una tecnica ampiamente utilizzata che ha applicazioni in una varietà di campi. Concepito per la prima volta nel 1984, è stato perfezionato nel 1988 (1, 2). L'obiettivo fondamentale dell'IP è la purificazione e l'isolamento di una proteina specifica utilizzando un anticorpo contro quella proteina. La parola "immuno" si riferisce all'uso di un anticorpo mentre la parola "precipitazione" si riferisce all'estrazione di una sostanza specifica da una soluzione. La proteina bersaglio potrebbe essere endogena o ricombinante. La maggior parte delle proteine ricombinanti ha un tag epitopo (cioè myc o bandiera) attaccato a loro per semplificare la successiva purificazione. In genere, è più facile ottimizzare la proteina IP ricombinante perché gli anticorpi contro i tag epitopi ricombinanti sono molto forti ed efficaci. Gli anticorpi contro le proteine endogene hanno un'efficacia estremamente variabile, rendendo molto più difficile ottimizzare queste IP. Un passo necessario dopo l'immunoprecipitazione è la verifica della purificazione. La proteina isolata viene risolta utilizzando SDS-PAGE e successivamente sonde per la purezza da western blots (Figura 1). Un controllo importante è l'uso di un anticorpo diverso durante il Western blot per verificare il pull down della proteina corretta. La combinazione di IP con tecniche successive è un potente strumento di analisi. L'obiettivo dopo la purificazione può essere la caratterizzazione della proteina stessa mediante NMR, spettrometria di massa e saggi in vitro, o l'analisi dei partner interagenti della proteina (cioè proteine, DNA, RNA) (3, 4, 5).
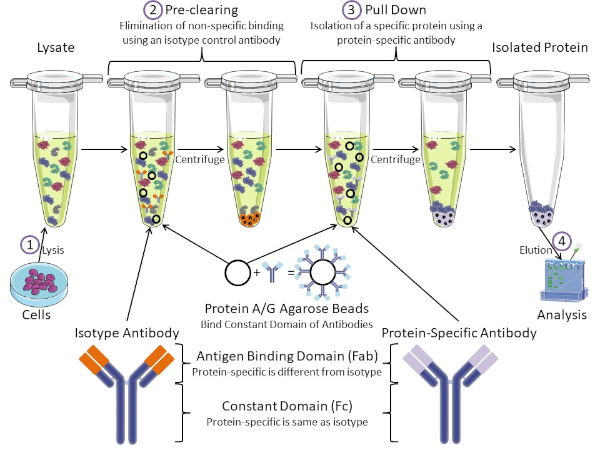
Figura 1: Panoramica della procedura di immunoprecipitazione. L'immunoprecipitazione è l'isolamento di una proteina specifica utilizzando un anticorpo. Dopo la produzione di lisi dalle cellule, ci sono due passaggi principali: pre-pulizia e pull down. Durante la fase di pre-clearing, i lasati cellulari viene pre-eliminata dalle proteine che si legano agli anticorpi in modo non specifico utilizzando un anticorpo di controllo dell'isotipo. Nella fase di pull down, la proteina bersaglio viene tirata verso il basso usando un anticorpo specifico della proteina. La proteina isolata viene quindi analizzata da Western blot. Gli anticorpi isotipia e gli anticorpi specifici della proteina hanno lo stesso dominio costante, ma domini di legame dell'antigene diversi. Un componente chiave di questo protocollo sono le perle di agarose della proteina A / G che legano il dominio costante degli anticorpi, consentendo l'immunoprecipitazione della proteina bersaglio. Fare clic qui per visualizzare una versione più grande di questa figura.
Gli anticorpi sono il componente chiave di un'immunoprecipitazione che la differenziano da altre forme di purificazione delle proteine (cioè la purificazione della colonna di affinità del nichel). Gli anticorpi sono molecole prodotte da cellule B in grado di riconoscere specifici epitopi proteici. Gli anticorpi hanno due domini: costante (Fc) e legame con l'antigene (Fab) (Figura 1). Il dominio costante identifica il tipo di anticorpo e detta la funzione in vivo. Di solito, i domini costanti degli anticorpi utilizzati per l'IP sono IgG di topo, ratto o coniglio. La porzione di legame con l'antigene dell'anticorpo riconosce un epitopo specifico di una proteina specifica. Gli anticorpi possono riconoscere gli epitopi su proteine ripiegate che potrebbero non esistere quando la proteina è denaturata e viceversa. Pertanto, la disponibilità dell'epitopo dipende dal ripiegamento delle proteine, identificando un fattore importante da considerare quando si scelgono anticorpi e condizioni per l'IP.
Sia il sistema procariotico che eucariotico hanno proteine leganti gli anticorpi. Nei sistemi eucariotici, lo scopo è la protezione immunitaria dai batteri mentre nei sistemi procariotici, lo scopo è la protezione dal sistema immunitario. Le proteine leganti gli anticorpi influenzano la metodologia IP in due modi. In primo luogo, c'è una fase di pre-clearing necessaria (Figura 1) per liberare il lisiato dalle proteine che legano gli anticorpi, riducendo così il legame non specifico nel prodotto finale. Questo passaggio utilizza un anticorpo isotipo che ha lo stesso dominio costante ma un dominio di legame anticorpale diverso rispetto all'anticorpo specifico della proteina. Le proteine che legano gli anticorpi batterici sono il secondo componente chiave di questo metodo. Dopo che l'anticorpo proteina-specifica lega la proteina bersaglio, il complesso anticorpo: proteina deve essere tirato verso il basso (Figura 1). Le proteine A, G e L sono proteine batteriche che legano il dominio costante degli anticorpi. Mentre i batteri usano questo per sovvertire il sistema immunitario, i ricercatori hanno cooptato questo sistema per una facile purificazione degli anticorpi, e viene utilizzato sia durante le fasi di pre-clearing che di pull-down. Queste proteine hanno diverse affinità di legame per diverse specie e diversi sottotipi di dominio costante - un altro fattore da considerare quando si scelgono le condizioni per IP. Molte aziende vendono perle di agarose marcate Protein A / G (Figura 1), colonne di spin prefabbricate o resine per realizzare colonne. In generale, le pere e le colonne di spin vengono utilizzate per campioni di dimensioni inferiori, mentre le resine vengono utilizzate per la purificazione alla rinfusa.
In questo esercizio di laboratorio, dimostriamo come purificare la proteina endogena c-myc, dai timociti murini primari, utilizzando la tecnica di immunoprecipitazione di base basata sulle perle di agarose Protein A / G Plus. Il protocollo inizia dalla preparazione del lizzata cellulare e termina con la verifica del successo del pull down proteico utilizzando l'analisi Western blot.
Procedura
1. Immunoprecipitazione con pere di agarose della proteina A/G PLUS
Preparazione del losato cellulare
- Centrifugare 108 timociti in una microcentrifuga a 13.000 giri/min per 3 minuti e rimuovere il surnatante.
Nota: Il numero di cellule varierà a seconda dei livelli di espressione della proteina desiderata e del tipo di cellula scelto. - Sospendere di ricaspendere le cellule nel tampone di lisi da 500 μ
Risultati
I risultati della procedura descritta sopra sono mostrati nella Figura 2. Da sinistra a destra, le corsie contengono il gruppo di controllo (isotipo), il gruppo di prova (c-myc), il lisirato pre-eliminato (lisi) e la scala di peso molecolare (scala). Le bande della scala da 25 e 75 kDa sono contrassegnate. Le due bande prominenti a ~ 25 kDa e 50 kDa sono rispettivamente la catena leggera e pesante dell'anticorpo legante e non sono specifiche per l'IP o i campioni. Proteina c-myc che corre
Applicazione e Riepilogo
In breve, l'immunoprecipitazione è l'isolamento di una proteina specifica utilizzando un anticorpo. In questo esempio, i risultati dell'immunoprecipitazione sono stati analizzati da Western blot per valutarne la purezza. La proteina isolata potrebbe essere utilizzata in una serie di applicazioni in seguito, tra cui: NMR per la struttura proteica, Spettrometria di massa per la sequenza di amminoacidi o saggi in vitro per la caratterizzazione enzimatica. Gli IP possono anche caratterizzare i partner interagenti d.
Riferimenti
- Olliver, C. L. and Boyd, C. D. (1984). Immunoprecipitation of In Vitro Translation Products with Protein A Bound to Sepharose. In J. M. Walker (eds), Nucleic Acids. Methods in Molecular Biology (pp. 157-160). New Jersey: Humana Press.
- Thurston, C. F. and Henley, L. F. (1988). Direct Immunoprecipitation of Protein. In J. M. Walker (eds), New Protein Techniques. Methods in Molecular Biology (pp. 149-158). New Jersey: Humana Press.
- Anderson, N. G. (1998). Co-immunoprecipitation: Identification of Interacting Proteins. In R. A. Clegg (eds), Protein Targeting Protocols.Methods in Molecular Biology (pp. 35-45). New Jersey: Humana Press.
- Jackson, D. I. and Dickson, C. (1999). Protein Techniques: Immunoprecipitation, In Vitro Kinase Assays, and Western Blotting. In P.T. Sharpe and I. Mason (eds), Molecular Embryology. Methods in Molecular Biology (pp. 699-708). New Jersey: Humana Press.
- Trieu, E. P. and Targoff, I. N. (2015). Immunoprecipitation: Western Blot for Proteins of Low Abundance. In B. Kurien and R. Scofield (eds), Western Blotting. Methods in Molecular Biology (pp. 327-342). New York, NY: Humana Press.
Vai a...
Video da questa raccolta:

Now Playing
Tecniche basate sull'immuno-precipitazione: purificazione di proteine endogene con l'impiego di microsfere di agarosio
Immunology
87.6K Visualizzazioni

Citometria a flusso e selezione cellulare attivata dalla fluorescenza (FACS): isolamento dei linfociti B della milza
Immunology
92.9K Visualizzazioni

Magnetic Activated Cell Sorting (MACS): isolamento dei linfociti T timici
Immunology
22.9K Visualizzazioni

Saggi ELISA: indiretti, sandwich e competitivi
Immunology
238.1K Visualizzazioni

EliSPOT Assay: Rilevamento di splenociti secernenti IFN-γ
Immunology
28.4K Visualizzazioni

Immunoistochimica e immunocitochimica: imaging dei tessuti tramite microscopia ottica
Immunology
78.8K Visualizzazioni

Generazione di anticorpi: produzione di anticorpi monoclonali attraverso l'utilizzo di ibridomi
Immunology
43.5K Visualizzazioni

Microscopia a immunofluorescenza: colorazione a immunofluorescenza di sezioni di tessuto incorporato in paraffina
Immunology
53.8K Visualizzazioni

Microscopia a fluorescenza confocale: una tecnica per determinare la localizzazione delle proteine nei fibroblasti di topo
Immunology
43.1K Visualizzazioni

Analisi del ciclo cellulare: valutazione della proliferazione delle cellule T CD8 e CD4 in seguito a stimolazione tramite colorazione CFSE e citometria a flusso
Immunology
24.2K Visualizzazioni

Trasferimento di cellule adottive: introduzione degli splenociti di topo donatore a un topo ospite e valutazione del successo tramite FACS
Immunology
22.3K Visualizzazioni

Saggio per la morte cellulare: saggio di rilascio di cromo della capacità citotossica
Immunology
151.4K Visualizzazioni