Method Article
Measuring the Structure, Composition, and Change of Underwater Environments with Large-area Imaging
In This Article
Summary
This protocol covers a four-step large-area imaging survey methodology used to extract metrics of structural complexity, community composition, and population demographics for coral reef communities. The quality of imagery collected and integrated access to the source imagery are prioritized within each step of the protocol.
Abstract
Digital imaging and processing technology have evolved to facilitate the expansion of large-area imaging surveys, which increase our capacity to study the status, trends, and dynamics of organisms living in subtidal habitats. By creating photo-realistic digital twins for ex situ analyses, these approaches allow small field teams to collect substantially more data than was previously possible. Here we present a four-step, large-area imaging survey pipeline and analysis methodology, including image collection, model construction, ecological analysis, and data curation, that has been developed and refined through experimentation over the past decade. Each step described has a consistent focus on the unique value of the original source imagery. While types of data extracted from large-area image surveys are vast, we include here workflows to extract ecological data for structural complexity, community composition, and demographic analyses valuable for monitoring and hypothesis-driven efforts. We additionally include recommendations for metadata standards, which complement the collection of large-area imaging data and support archival efforts facilitating transparency and collaboration between research groups.
Introduction
Across terrestrial environments, researchers have taken advantage of standardized large-area sampling of ecological communities, particularly in the context of long-term study sites, including Barro Colorado Island1, the Hubbard Brook experimental forest2, and others3. Through the collection of spatially explicit and taxonomically resolved distributional data, such sampling has been used to explore fundamental ecological dynamics, such as dispersion and recruitment patterns3,4,5, habitat preference and availability, dispersal kernels, resource limitation3,5,6,7,8, and space use9,10. However, to date, most spatial studies of marine communities have relied on metrics of relative cover, reported as percent cover occupied by taxon or group11,12,13,14,15. Aggregated estimates of relative cover, however, are insufficient to resolve details of population-level demography as well as community-level dynamics. Studies that have provided detailed analyses of benthic communities have relied upon laborious in-water monitoring protocols16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32, but the scale (including taxonomic, spatial, and chronological scales) of these studies is notably limited due to the operational demands of in-water methodology.
Large-area imaging (LAI) is an approach that combines information from numerous images through computationally intensive workflows to create photo-realistic representations of environments at scales much larger than that of the constituent images33. The LAI workflow is particularly well-suited to applications in underwater habitats given the limited visibility due to light absorption and scattering in water. Because of the limited visibility, imagery that captures fine details of the benthos must be acquired near to the subject; to capture a landscape (or seascape) view of a broad swath of benthic habitat while retaining fine detail of individual benthic subjects thus requires composite imaging. Further, in structurally complex environments, it is essential to account for the three-dimensional (3D) structure in reconstructing the composite imaging to produce faithful representations of the position and relative proximity of benthic organisms. The Structure-from-Motion (SfM) photogrammetric method has been applied to environments with relatively immobile benthic organisms, including coral reefs34,35,36, Antarctic benthic ecosystems37, cold-water coral reefs38, cold seeps39, and seagrass habitat40, generating composite imaging without stereoscopy used to reconstruct a landscape scene with follow-on ortho-map generation and point cloud estimation.
In coral reef science, LAI has offered the potential to visualize reefscapes at increasingly large spatial scales and to share these visualizations across digital media. LAI can be used to estimate the coverage of reef organisms, the density and distribution of coral colonies, as well as the shape and condition of individual organisms41,42,43,44,45,46,47. Further, when LAI products are collected from the same location at different points in time, it is possible to record changes in the size and condition of individual organisms48,49,50,51. Given that most scleractinian coral colonies grow on the order of millimeters to centimeters radially per year, time-series LAI collected across years can provide an invaluable data stream for reporting on the biology and ecology of these species52. Repeated and co-registered LAI data offer unique insights with which to study coral reefs in a format that can be shared, archived, and used as a basis for collaboration worldwide.
As the use of LAI has expanded among coral reef ecologists53, so has the diversity of camera systems and survey methodologies52. A chosen LAI protocol should target the resolution and scope of desired ecological metrics while staying bounded within available resources. The quality of any photogrammetric reconstruction will ultimately be dependent on the resolution of the source imagery and the spatial coverage of the survey area. Image quality is determined by an influence of camera parameters including sensor resolution and focal length, as well as the collection procedure, principally the distance from the benthos54, all of which contribute to the effective Ground Sampling Distance (GSD) of a particular set of images. Additionally, fast shutter speeds, small apertures, and low ISO values will produce images that are sharp, in focus, and have low electronic noise, respectively. Keeping each of these settings at thresholds that produce sufficient quality imagery can be a challenge in underwater environments where light levels are low. Larger sensors, like those found on digital single-lens reflex (DSLR) style and mirrorless cameras, generate better image quality and, in turn, more accurate reconstructions in comparison to smaller, more mobile solutions like action cameras55. Additional features that should not be overlooked when considering an appropriate camera model include a built-in intervalometer and sufficient storage and battery capacity to support extended-duration image collection efforts in the field.
Survey design should be driven by the ecological hypothesis, with the candidate metrics determining necessary resolution and spatial coverage. Within coral reef ecology, LAI has been used to characterize structural complexity35,36,56,57,58,59, community composition and assemblage60,61,62, spatial distribution45,63,64,65,66, and community trajectories48,49,50,67,68,69. The resolution of image quality should be appropriate to ecological data needs, with finer scale resolution on the sub-mm detail necessary to support polyp scale observations of competition along colony borders70 or surveys of small juvenile corals66,71. In contrast, extracting broad-scale habitat and structural metrics for coastal mapping72,73,74 requires a greater spatial extent with a reduced need for resolution at the cm-m scale. The demand for resolution must be balanced with the spatial extent required to obtain sufficient sampling and operational limits of the time needed to complete an LAI survey33.
Described here is an end-to-end protocol for conducting an LAI survey, which focuses on maximizing the quality, utility, and value of the source imagery, breaking down the protocol into four major steps: image collection, model construction, ecological analyses, and data curation33. The collection of approximately 3,500 LAI image surveys of over 2,000 unique reef sites over the past decade has contributed to the refinement of the methodology for each step presented here (https://doi.org/10.6075/J0T43RN1). The resulting protocol is a method for robust data collection and accurate and precise model reconstructions, which enable the collection of detailed ecological data across a broad range of applications, including structural complexity, community composition, and population demographics (e.g., density and size structure). We additionally include metadata standards for archiving LAI data, the establishment of which is essential to assure the preservation, transparency, and collaborative potential of these digital twins.
Protocol
1. Image collection
NOTE: The following large-area image collection procedure outlines a method to survey an area approximately 100 m2, though it can easily be adapted for areas ranging from 10 m2 up to 2,500 m2. The survey method described below is designed to be deployable in a variety of working conditions, generate high-quality imagery, and provide robust data that can be used for many ecological applications from the effort of a single hourlong dive by a two-person buddy team.
- Gear preparation
- Assemble the camera frame by attaching outer frame panels to camera mounting panels and columns using 1 1/2" long Philips flat head screws (Figure 1).
- Prepare two DSLR cameras, one with a fixed wide-angle lens and a second camera equipped with a zoom lens. See Table 1 for detailed camera settings.
NOTE: While the second zoom lens camera is optional, it is highly recommended to capture greater image resolution for determining details of taxonomy75,76, finding and identifying small juvenile corals66,71 (1-5 cm diameter), and distinguishing coral colony borders45,48,50 during future ecological analyses. - Assemble underwater camera housings by attaching the dome port and secure with included dome thumb screws. Attach the handles using 1/2" long Phillips head screws and the camera mounting plate using a 1 1/8" long socket head screw. Insert the cameras into the housing and use the vacuum pump to set the housing pressure to 5 in.Hg, checking the integrity of the o-ring seal.
- Install the housings on the camera frame by sliding the camera mounting plate onto the mounting frame panels and secure in place using thumbscrews.
- Plot setup
- Establish the boundaries of the plot using six square stainless steel tiles (10 cm side length) with printed coded markers. Place two central tiles 10 m apart along the target isobath, with four corner tiles added 5 m inshore and offshore of the center tiles to create a 10 m by 10 m square plot area (Figure 2). Record the depth at each of the six tiles to provide orientation to local vertical and facilitate subsequent model construction steps.
- Add reference floats with ~1.5 m of line attached to 0.45 kg (1 lb) weights approximately 1 m outside of each of the four corner tiles.
NOTE: These reference floats serve as a guide for the camera operator to denote the boundaries of the plot and the height above the benthos at which they should swim the camera. Placing them outside of the 10 m x 10 m plot boundaries creates a buffer zone of the imagery, helping to ensure the core imaged area is fully reconstructed. - Place a 0.5 m length scale bar consisting of two coded target tiles affixed to polyvinyl chloride (PVC) or aluminum bar in each quadrant of the plot for a total of four deployed scale bars.
NOTE: It is important that scale bars and depth tiles remain stable throughout the image collection process. Scale bars and depth tiles can also be used as visual landmarks for the camera operator to keep track of their swim pattern progress. - To establish permanent plots, install 0.46 m (18 in) stainless steel stakes (0.95 cm or 3/8 in threaded rod) secured with two-part marine epoxy using a mallet adjacent to each center tile to mark the center line of the plot. When looking onshore, the left stake includes a lock nut to assist with orientation during future survey efforts. At the end of the survey, record the Global Positioning System (GPS) coordinate directly above the left-center stake location using a GPS unit stored inside a waterproof case affixed to a dive float.
NOTE: Installation of permanent stakes is recommended when allowed through permitting to reduce search time on subsequent surveys and affirm resurveying the same reef area. Use a printed 2 Dimensional (2D) ortho-image or "roadmap" (Supplemental File 1) of the survey site to locate invariant features and assist with identifying the location of the same plot area for imaging. These roadmaps can also be used to relocate exact survey plots in instances where permitting does not allow the installation of permanent stakes or when stakes have been dislodged or removed.
- Image capture
- Set the custom white balance of each camera using a grey card at the targeted depth of the plot.
- Start each camera on an intervalometer set to capture on a 1 s-1 interval.
- Swim the camera system approximately 1.5 m above the benthos in a gridded pattern, followed by a second perpendicular gridded pass with approximately 1 m between each pass swimming at a slow speed of approximately 0.25 m s-1 (Figure 3). Ensure that the passes extend a minimum of 2 m beyond the edges of the plot boundaries to ensure sufficient overlap within the target plot area.
NOTE: Following this swim pattern and speed results in the minimum collection of approximately 1,700 images from each camera during 28 min of imaging. However, to account for turnaround time between passes and erring on the side of oversampling to buffer against insufficient overlap that results in suboptimal data, we recommend divers target the collection of 2,500 images from each camera during approximately 40 min of imaging. - Hold the camera system approximately perpendicular to the ocean surface to ensure sufficient top-down coverage; however, for survey sites with complex topography, adjust the camera orientation on the second pass to follow perpendicular to the benthos and reduce occlusions in the 3D reconstruction. Take care while angling the camera to minimize blue water that is captured in the imagery.
NOTE: In instances of suboptimal ocean conditions such as strong currents or steep slopes, both passes can be done in the same alongshore direction. Using just the single wide-angle lens camera may be necessary in strong current to reduce the drag profile and effort of the diver needed to maintain forward progress. The second set of passes should ideally be rotated slightly relative to the first. If two passes are done in the same alongshore direction, at least one or two perpendicular or diagonal "tie lines" cutting across the initial set of passes should be added to improve reconstruction quality.
2. Model construction
NOTE: The model construction step focuses on maintaining access to high-resolution source imagery and generating the derived dense point cloud. Referencing of the dense point cloud occurs within the centralized visualization and analytical software (see Table of Materials)77, allowing the user to input and modify on the fly. This removes the need to reprocess and re-export data products for a dataset when new information arises, particularly with additional surveys across time. 2D orthomaps, referred to here as orthoprojections, are generated using an orthorectified projection view of the dense point cloud, with the projection angle orthogonal to the direction of gravity.
- Camera alignment and 3D dense point cloud construction
- Using a high-performance computer, load all images, including those from the wide-angle and macro lens cameras into an Agisoft Metashape project by selecting Workflow | Add Folder. Once the files have loaded, select the data layout as Single cameras, Add all images to one chunk. Remove images with excessive blue water in the scene from the project.
NOTE: Before addition into the project, images from each camera should be organized into separate folders, which will separate image files within the chunk as distinct camera groups. - Align all images by selecting Workflow | Align Photos. See Table 2 for processing settings for alignment.
- Once the alignment is complete, verify that the image set has successfully aligned based on the percentage of cameras aligned and inspection of the generated sparse point cloud for gaps in coverage or misalignments. Ensure the bounding box encompasses the entire sparse point cloud before proceeding. If necessary, modify it using the Resize or Rotate Region options.
NOTE: It is possible to have 100% of images align and still have gaps in parts of the model and conversely have 80-90% of images align but have a complete model of the target survey area. Therefore, both percentage of alignment and areal coverage should be used to make an informed decision on the usability, in part or in whole, of the generated data set or whether additional efforts in increasing image acquisition quality or adjustments to alignment processing settings are needed. - Disable the camera group containing the zoom lens images. Construct the dense point cloud by selecting Workflow | Build Dense Cloud. See Table 2 for processing settings for Build Dense Cloud.
NOTE: While images from both cameras should be used for alignment to facilitate query of all high-resolution images during ecological analyses, zoom lens images should not be used during creation of the dense point cloud, as the subtle differences in white balance and exposure between the two cameras will add visual noise to the dense point cloud. - Export the camera pose estimates by selecting Tools | Run Script | Extract_meta.py script (Supplemental File 2). Export the Dense Point Cloud by selecting File | Export | Export Points. See Table 2 for settings for export.
- Drag and drop the exported dense point cloud file onto the vc5prep-confidence.bat file located within the visualization software's program files.
- Compile the exported data files, including the camera pose files (*.cams.xml and *.meta.json) along with the generated program files (*.vml and folder containing *ptdata, *.xml, *.kdm files) into a single directory for use in the visualization software.
- Using a high-performance computer, load all images, including those from the wide-angle and macro lens cameras into an Agisoft Metashape project by selecting Workflow | Add Folder. Once the files have loaded, select the data layout as Single cameras, Add all images to one chunk. Remove images with excessive blue water in the scene from the project.
- Scale and orientation
- In the visualization software, use the scaler tool to place marker pairs on scale bar targets and input the known distance.
NOTE: Further details on the scaler tool can be found in section 4.2 of Supplemental File 3. - Place markers on each depth tile and use the orient tool to adjust depth values for each tile to define the best fit plane to the local vertical.
NOTE: Further details on the orient tool can be found in section 4.4 of Supplemental File 3.
NOTE: Verify that the depth rays point upwards, ending at the estimated water surface. In the event of downward-facing rays, check for evidence of movement in depth tiles that would lead to errors in the depth value relative to its final reconstructed location or errors in depth metadata.
- In the visualization software, use the scaler tool to place marker pairs on scale bar targets and input the known distance.
- Temporal coregistration
- Create an org project file in the software for a collection of data following section 10.0 of Supplemental File 3. Include multiple sites with surveys through time in a single org project.
- Add dense point cloud files to the org project as layers and modify the site level organizational structure as needed to connect dense point clouds of a given site across time.
NOTE: Use a consistent file naming scheme of [Region]_[Date]_[Site] to automate layer organization. - Select a time point for a site to serve as the reference layer for scale and orientation of the time series.
NOTE: Multiple layers can be used to set scale; however, one time point layer must be selected as reference for orientation, as tidal shifts and large swell height lead to error in the precision and consistency of depth measurements across time. - Use the assisted coregistration tool to align layers across time.
NOTE: Further details can be found in section 11.0 of Supplemental File 3. In cases of large structural change due to high growth or big swell events, it may be preferable to use the manual coregistration workflow using candidate invariant features. For longer time series, or when there is substantial change through time, it is generally recommended to coregister a given model to the next earlier time point.
- 2D Orthorectification
- Using the boxes tool in the software, set a view from a surface angle (g: 0°) with boundaries encompassing the focal analysis area (10 m x 10 m) along with a minimum 2 m buffer (minimum w: 14 m, minimum h: 14 m) extending from each edge.
NOTE: Details for using the boxes tool can be found in section 6.0 of Supplemental File 3. - Within the boxes tool, turn on map export to create an orthoprojection image file. Set the export resolution to be 1 mm px-1 and select capture to generate a preview. Scroll to a preview tile containing a complete section of the dense point cloud and increase the pt-size value to fill in gaps between points.
- Select capture to export the orthoprojection as a ppm file. Once capture is complete, convert the exported file to a tif by dragging and dropping the generated .ppm file onto the convert-to-tif-flip.cmd file located within the program files.
- For coregistered dense point clouds of a time series, repeat steps 2.4.2 to 2.4.3 for the site, changing the time layer while using the same box.
- Using the boxes tool in the software, set a view from a surface angle (g: 0°) with boundaries encompassing the focal analysis area (10 m x 10 m) along with a minimum 2 m buffer (minimum w: 14 m, minimum h: 14 m) extending from each edge.
3. Ecological analysis
NOTE: There exist myriad options for ecological data extraction, a selection of which we present here. These core workflows focus on established metrics for long-term monitoring78,79 but can be used and adapted to generate data sufficient for observation-based scientific inquiry. Users should select and adapt workflows based on their individual data needs and analytical goals. The workflows described below are designed to integrate direct access to the source imagery to aid in the annotation of biological data, using derived products such as the 3D dense point cloud or 2D orthoprojection as an organizational framework.
- Structural complexity
- Using the rugo tool, make a 10 m x 10 m box on the dense point cloud, setting a maximum dimension of 10 m (rugo-dim: 10.0 m) and aspect ratio of 1.0 (quad-aspect: 1.000) to designate the 100 m2 target area for data extraction.
NOTE: Further details on using the rugo tool can be found in section 7.0 of Supplemental File 3. - Set the number of transect lines to sample (lines) and the number of points along each transect (samples) based on the desired sample spacing. Select prepare to export a csv file containing x, y, and z coordinates of each sampled point, which can be used for a variety of structural complexity analyses.
- Run the script found in Supplemental File 4 to generate functions used for structural complexity analyses. Then, follow the scripts found in Supplemental File 5 based on the desired metrics to quantify structural complexity.
NOTE: It is suggested to select a spacing scale at the highest resolution (1 cm spacing recommended) users may target to address their scale of interest, from which a variety of scales, such as those presented here (0.5 m line spacing and 10 cm point spacing), can similarly be assessed through downsampling56.
- Using the rugo tool, make a 10 m x 10 m box on the dense point cloud, setting a maximum dimension of 10 m (rugo-dim: 10.0 m) and aspect ratio of 1.0 (quad-aspect: 1.000) to designate the 100 m2 target area for data extraction.
- Community composition
- Using the Virtual Point Intercept (VPI) tool, make a 10 m x 10 m box on the dense point cloud, setting a maximum dimension of 10 m (quad-dim: 10.0 m) and an aspect ratio of 1 (quad-aspect: 1.000) to designate the target 100 m2 area for data extraction.
NOTE: Details on using the Virtual Point Intercept tool can be found in section 5.0 of Supplemental File 3. - Set the points to be sampled from the dense point cloud in a stratified random distribution, with the number of points targeting a chosen density. Select prepare to begin sampling points.
NOTE: A sampling density of 25 m-2 (2,500 points) is recommended for analyses at the taxonomic level. The results presented here used a lower sampling density (10 m-2) for a broader community composition comparison survey focused on a functional level. - Use the cams tool to link the source images to the dense point cloud and allow spatially queried multi-image views of points on the model.
NOTE: Further details on the cams tool can be found in section 4.5 of Supplemental File 3. - Use the VPI browser web applet to primarily label each point with its highest resolution taxonomic designation directly using multiple views of the source imagery. Add two optional sets of secondary labels for each point, with examples for coral labels including bleaching stress75 and morphology.
NOTE: Primary and secondary label sets can be modified by editing the qclasses.json file located in the *.pq folder. - Export a summary of the percent cover for each label as a .csv file using the web applet.
- Using the Virtual Point Intercept (VPI) tool, make a 10 m x 10 m box on the dense point cloud, setting a maximum dimension of 10 m (quad-dim: 10.0 m) and an aspect ratio of 1 (quad-aspect: 1.000) to designate the target 100 m2 area for data extraction.
- Density survey
- Ensure the images have already been linked in the software following step 3.2.3. Set a pseudo-map view of the dense point cloud by changing the focal length of the perspective view to 100 mm and zooming out to a top-down, full view of the model. Use the quadrat sampling file in Supplemental File 6 to capture the view using the web applet by clicking eval for cell c1, then select the grab button.
NOTE: Further details can be found in section 8.0 of Supplemental File 3. - Turn cams on, then link images within the quadrat sampling workflow by clicking eval for cells c2 and c3 in the quadrat sampling script.
- Ensure a rugo box has already been made following step 3.1.1 to designate the target 100 m2 data extraction area. In the web applet, eval the c4 prep cells section to sample 100 1 m2 quadrats.
- In the Quadrat Sampling web address, use the source imagery to search through a quadrat and label target organisms. Double left-click on a location to retarget the sampling location. Click a taxonomic button to designate the targeted point as a sample. To remove a marked point, double left-click that point and select NOTHING.
NOTE: Quadrats are presented in a predetermined random order, allowing a subset of quadrats to be randomly sampled for density surveys. - Compile all sampling files located under *aux/recruits/test1 into a single directory, renaming each file to include the site name. Add the button lookup file (Supplemental File 7) to the same directory as the sampling files. Run the script in Supplemental File 8, following in-line instructions to aggregate sample data into density by site and taxonomic group.
NOTE: Here, we sample sessile invertebrates, but the same tool can be used to survey a variety of organisms, including juvenile and adult coral densities.
- Ensure the images have already been linked in the software following step 3.2.3. Set a pseudo-map view of the dense point cloud by changing the focal length of the perspective view to 100 mm and zooming out to a top-down, full view of the model. Use the quadrat sampling file in Supplemental File 6 to capture the view using the web applet by clicking eval for cell c1, then select the grab button.
- Demography
- Load the orthoprojection file for colony segmentation in the segmentation analysis software (see Table of Materials)80. Load coregistered orthoprojection files for multiple time points as new maps within the same project file to segment and track the size of colonies through time.
- Use the source imagery via the web applet for iView found in section 17.1 of Supplemental File 3 while segmenting colonies as a reference for taxonomic and boundary identification. Ensure the images have already been linked in the visualization software following step 3.2.3. Place a marker on the dense point cloud for the focal colony and scroll through source images for the marker location for different perspective views.
- Use the positive/negative clicks annotation tool to segment individual coral colonies. Modify boundaries using click-based or manual boundary adjustment.
4. Data curation
NOTE: Archival efforts should prioritize preservation of the source imagery, as all subsequent derived products are reproducible. While repositories available to a given user will vary, efforts should be made to standardize associated survey metadata included within the source image dataset to maximize their utility when made available to the broader community.
- Data repository
- Generate a methods description file that includes survey details such as area covered, camera system, ground control markers, and collection pattern. See Supplemental File 9 for an example description for this protocol.
- Generate a survey metadata file specific to the image dataset, including fields such as site name, collection date, GPS coordinates, plot bearings, ground control depth and scale data, and the used collection pattern and camera system for the given survey.
NOTE: Additional fields which include broader geographic context and conditions during the survey are highly recommended. An example metadata file with recommended fields can be found in Supplemental File 10. - Combine the description file, metadata file, and image files into a single zip archive to be ingested into the chosen data repository.
NOTE: Collections of image data have been made available at https://doi.org/10.6075/J0DV1HDR.
Results
Successful large-area image collection in the field should result in the creation of a dense point cloud reconstruction with full top-down coverage of the survey area, while inadequate redundancy in coverage may result in gaps or full degradation of the point cloud (Figure 4). For a set of 43 large-area image surveys conducted across the Hawaiian archipelago in 2016, an average of 99.6% of images were aligned per dataset, with 66% of image sets having 100% of images successfully aligned. Images collected from the wide-angle lens camera had an average GSD of 0.52 mm px-1 while images from the zoom lens camera had an average GSD of 0.18 mm px-1. These surveys generated dense point clouds with an average size of 557.7 million points (15 GB).
The ecological workflows described here were designed to generate data comparable to existing methodologies for coral reef monitoring78. Ecological data extracted from the LAI surveys across Hawaii show, apart from a few outliers, measures of linear rugosity resulting from the structural complexity workflow which align well with relative values compared to in situ measures of complexity across sites81 (Figure 5A,B). Additional community composition analyses from LAI to measure the percent coverage of major benthic functional groups show similar alignment compared with traditional photoquadrat surveys82 (Figure 5C,D). Quadrat sampling here was used to measure the density of sessile invertebrates, the most common being sea urchins, which were summarized as categorical measures of relative abundance. LAI methods regularly recorded higher levels of abundance relative to in situ methods81 (Figure 5E,F), which may be due to the ability to comprehensively search and survey all individuals within a given area compared with a rapid visual census. Segmenting coral colonies using the 2D orthoprojection also revealed similar size distributions of common coral taxa to in situ methods83 (Figure 5G,H).
A major advantage of LAI surveys is the ability to archive and track changes in reef areas across time through coregistration of the dense point cloud. Coral reefs are living substrates, which for LAI surveys means it can be challenging to identify permanent surfaces, either natural or installed, that can be used to reliably coregister dense point clouds from different time points. The example from Millennium atoll (Figure 6) shows an example with both high growth and structural loss where the assisted coregistration workflow was used for dense point cloud coregistration despite little to no stability of the reef surface across time.
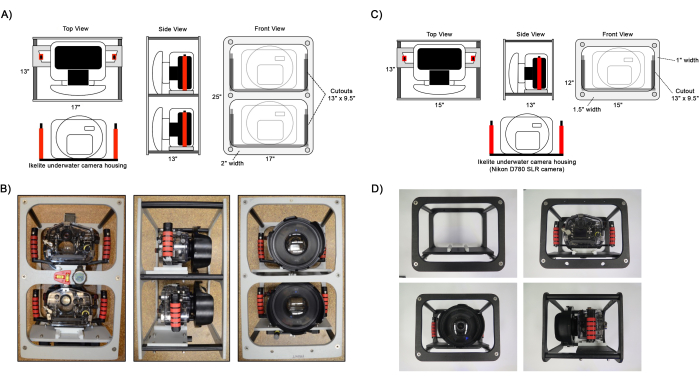
Figure 1: Diagram of assembled camera frames. Example views of the (A,B) dual and (C,D) single camera frame setups. Ikelite camera housings are attached to the frame on the camera mounting panel using a slider plate attached to the housing handles. Optional instruments to aid in navigation such as a level, compass, and dive computer can be attached to the frame as shown in B. Please click here to view a larger version of this figure.

Figure 2: Diagram of set up 100 m2 large-area image plot. Schematic of a fully set up large-area image plot 100 m2 in area. Temporary plot markers include six boundary tile markers, four scale bars, and four reference floats. Permanent plot markers include two stainless steel stakes with the left stake, when looking onshore, including a lock nut. The GPS reference for the plot should be taken above the left-center tile or stake. Please click here to view a larger version of this figure.
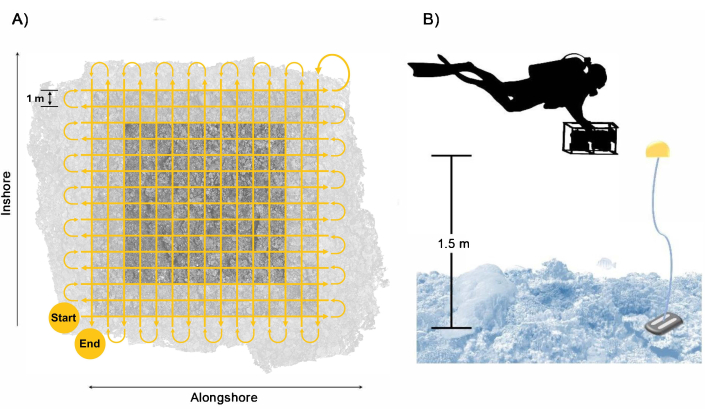
Figure 3: Diver collection pattern. Example of (A) swim path of diver to ensure sufficient coverage and overlap of the plot area with a buffer included and (B) arrangement of the diver with the camera system with the reference floats providing a guide for the swim height. Please click here to view a larger version of this figure.

Figure 4: Reduction in image overlap. The degradation of the dense point cloud as the number of overlapping images is reduced through random sampling. Each panel shows the dense point cloud generated from (A) all images, (B) ½, (C) 1/3, (D) 1/5, and (E) 1/10 of the original images. Please click here to view a larger version of this figure.

Figure 5: Ecological data extraction. Candidate data extracted from large-area imaging for each of the ecological workflows are shown in comparison to established in situ data collection methods. This includes (A,B) structural complexity, (C,D) community composition (error bars indicate standard error), (E,F) invertebrate density, and (G,H) size structure demographics. Abbreviation: LAI = large-area imaging. Please click here to view a larger version of this figure.

Figure 6: Dense point cloud coregistration. An example visual comparison of two temporally coregistered dense point clouds. Areas of structural loss, likely from swell damage, are visually coded in red, as indicated by the magenta arrow. Areas of structural addition, typically attributed to growth of coral colonies, are visually coded in blue, as indicated by the yellow arrow. The coregistration workflow described here can still be used for reef areas as dynamic as seen here, where permanently installed Ground Control Points (GCPs) would be unreliable due to overgrowth or dislodgement. Please click here to view a larger version of this figure.
| Camera Feature | Recommended Setting |
| Focus | Auto |
| Shooting Mode | P (Programmed Auto) for wide-angle lens |
| A (Aperture-priority auto), set to an aperture of F8 for macro lens | |
| Release Mode | S (Shutter-priority Auto) |
| Autofocus Settings | Autofocus central (AF-C), Single Central Point (S) |
| Auto ISO Sensitivity Control | ON |
| Maximum ISO Sensitivity | 3200 |
| Minimum Shutter Speed | 1/320 |
| Image Quality | RAW + JPEG |
| Interval Timer | 1 s |
| White Balance | Custom |
Table 1: Recommended camera settings. The following is a list of key camera settings used to optimize image quality. These settings prioritize the capture of in-focus images captured by a moving operator in underwater lighting conditions.
| Align Photos | |
| Accuracy | High |
| Generic Preselection | No |
| Key Point Limit | 5000 |
| Tie Point Limit | 0 |
| Guided Image Matching | No |
| Adaptive camera model fitting | Yes |
| Build Dense Cloud | |
| Quality | High |
| Depth Filtering | Mild |
| Calculate Point Colors | Yes |
| Calculate Point Confidence | Yes |
| Export Points | |
| File Type | Stanford PLY |
| Coordinates System | Local Coordinates (m) |
| Source Data | Dense cloud |
| Save point colors | Yes |
| Save point normal | Yes |
| Save point confidence | Yes |
| Save point classes | No |
| Convert colors to 8 bit RGB | Yes |
| Binary encoding | Yes |
Table 2: 3D Dense point cloud construction settings. A list of settings used in Agisoft Metashape to create and export a high-quality dense point cloud reconstruction.
Supplemental File 1: Roadmap. Example orthomosaic image marked with plot features and depths to aid in finding plot area for resurvey. Please click here to download this File.
Supplemental File 2: Extract_meta.py. Script run in Agisoft Metashape to export camera pose and file directory information for use in Viscore to query the original images. Please click here to download this File.
Supplemental File 3: Guide to Viscore. Software guide for Viscore, which includes workflows for model visualization, coregistration, and ecological analyses. Please click here to download this File.
Supplemental File 4: Rugosity_Functions.Rmd. Script used in R containing functions to process rugosity data extracted from Viscore. Please click here to download this File.
Supplemental File 5: Rugosity_Analysis.Rmd. Script used in R to calculate rugosity metrics. Please click here to download this File.
Supplemental File 6: Quadrat_sampling.rpl.json. Script used in Viscore for quadrat density analysis workflow. Please click here to download this File.
Supplemental File 7: Density_taxo_lookup.json. Button lookup file for running quadrat sampling script to aggregate quadrat sampling data by taxonomic group. Please click here to download this File.
Supplemental File 8: Density_Analysis.R Script used in R to aggregate quadrat sampling data that calculates density by taxonomic group at a survey level. Please click here to download this File.
Supplemental File 9: README.txt Example text file to be included with original imagery for data archiving that describes image capture methodology. Please click here to download this File.
Supplemental File 10: METADATA_KAH_2016-07_03.txt Example text file to be included with original imagery for data archiving that contains metadata fields for LAI survey. This includes fields for within survey scale and depth data for referencing as well as overall site metadata for geographic context. Abbreviation: LAI = large-area imaging. Please click here to download this File.
Discussion
Large-area imaging is a tool enabling domain scientists to digitally visualize and analyze features of the environment at scales larger than that of the individual images collected. By capturing multiple images of the environment from multiple perspectives, LAI protocols help to create representations of relatively broad landscapes (relative to the spatial coverage of individual images) while maintaining the detail collected from the original imagery. The unique value of LAI lies in the ability to explore environmental data across scales, from the largest scale (defined by the areal extent of the survey) to the finest scale (defined by the realized resolution of the original imagery). However, to capitalize on this cross-scale strength, it is critical to assure regular and fluid access to all levels of the data captured, specifically in assuring facile access to both the original imagery and the derived 3D model. In each step of the protocol presented here, we highlight this unique strength of LAI by consistently assuring that the original imagery is accessible, usable, and securely archived alongside the derived LAI models.
The LAI method will deliver products that are linked directly to the original imagery collected. By shifting details of image acquisition, users can produce data products of differing quality and coverage. When surveying structurally complex coral reef environments, a user with limited survey time underwater (or a constant number of images available to be captured) may prioritize increasing the areal coverage of the survey area or increasing the level of detail of each section of the area sampled. There will necessarily be a tradeoff, with the large areal model having less detail (and perhaps more occlusions) per unit area and the detailed model covering less total area (with likely fewer occlusions). In this protocol, we include the use of two cameras, each with different lenses, which enables a user to sample a larger area (sufficient overlap with wide angle lens to fulfill SfM requirements) while simultaneously collecting more detailed original imagery (higher detail from zoom lens which has less photo-to-photo overlap). By including pose estimation of imagery from both cameras, the downstream visualization and analysis protocols include higher-resolution views from much of the sampled area. Although the protocol aims to expand the range of survey viability, users may find that the derived products lack sufficient areal coverage or sufficient detail of original images to complete preferred analytical routines. Users are encouraged to review the original imagery and the derived models to ensure that the environmental survey protocol meets program needs and to modify the in-water survey approach (e.g., shifting ground sampling distance, altering survey duration or number of images collected) to arrive at the preferred balance of areal coverage and resolution per unit area.
LAI methods provide value to underwater science by capturing information-rich, wide-coverage 'snapshots' of benthic environments that can be time-efficient and require limited domain-specific expertise for collection. The value of these data products can be considered with reference to existing data streams as well as for novel and accelerated domain-specific applications. Considering the comparison with existing data streams, analysis products from LAI can provide ecological data that are directly comparable to data collected in situ by underwater observers84,85,86,87. We provide here a quantitative analysis of ecological data outputs derived from each, classical in situ monitoring activities and from standardized analysis of LAI products, following this protocol. By focusing on four common metrics in coral reef monitoring efforts (structural complexity, benthic community composition, density of mobile invertebrates, and coral size structure; Figure 5), we demonstrate strong quantitative concordance in data outputs. Notably, for the data streams that require fine-scale observations (e.g., taxonomic identifications, precise definitions of biological borders), LAI workflows that include regular and reliable access to original imagery provide unique strength to parallel the observational opportunities that have historically been limited only to immersive, in situ sampling. Advances in data handling and visualization provided by Viscore and described in this protocol offer unique value in assuring the comparability of LAI-derived ecological data and in situ monitoring products, allowing for unbiased maintenance of long-term data streams with the incorporation of digitally enhanced workflows of LAI.
For underwater scientists, LAI offers the opportunity to leverage novel and accelerated workflows in data acquisition and exploration. LAI has unique strengths as a high-resolution mapping tool that contains information not just describing the relative composition of the benthic environment but also spatial characteristics. Explicit to the creation of a 3D model from the SfM workflow, LAI products contain information about the structural complexity which can be explored across multiple scales56. As tools capturing larger-area seascapes, LAI products can provide an opportunity to consider patterns of spatial distribution and neighborhood characteristics for benthic organisms45,66. Moreover, by being able to visualize benthic landscapes at larger scales, it becomes possible to detect features that are not readily visible in underwater habitats due to the limitations of long-distance visibility underwater, for example large-scale (3-4 m) polygonal patterning of a common macroalga on a Pacific coral reef64.
While LAI provides opportunities for large-scale analysis, there have been stated concerns regarding the challenges linked with efficient in-water collection and image post-processing. Expanding the spatial extent of image acquisition underwater will demand technological advances away from diver-deployed imaging, to the utilization of assisted mapping systems88, and ultimately the use of ROVs38 and AUVs67,89. Robust survey methodologies in regard to the acquisition pattern and camera systems will ensure a smooth transition and consistency in the data generated across these different platforms. Given the computational demands as well as the large data format of LAI products, some marine scientists have expressed concerns regarding technological accessibility of the workflow90 and the large time demands linked with ecological data extraction84,86. A growing number of tools are being presented, however, that leverage creative solutions to the technical hurdles of data extraction80,91,92,93. Importantly, the use of AI-enhanced workflows of LAI analysis is limited by the quality of the input signal provided. As such, there remains a consistent demand in maintaining standards and quality of image acquisition and data management in LAI protocols regardless of whether the data extraction is conducted by a human observer, by a trained AI algorithm, or (ideally) by an AI-accelerated, human-in-the-loop workflow. By maintaining a consistent focus on the primary importance of the original imagery in the LAI protocols, as described here, unique opportunities emerge to explore underwater habitats robustly, transparently, and consistently.
Disclosures
The authors have no conflicts of interest to disclose.
Acknowledgements
This work is supported by the efforts of the 100 Island Challenge at Scripps Institution of Oceanography. We thank Schmidt Marine Technology Partners, Ed and Christy Scripps, and the Moore Family Foundation for their financial support of the associated research and large-area imaging training efforts which have helped to refine the methodology. In addition, we thank the crew of research vessels Hi'ialiki, Hanse Explorer, and Plan B, who provided support for field efforts. We particularly thank the team at the Ecosystem Sciences Division of the Pacific Islands Fisheries Sciences Center at NOAA who assisted with field collection of the data presented here.
Materials
| Name | Company | Catalog Number | Comments |
| 1" x 4 1/4" x 3 1/4" custom machined acetal | N/A | N/A | 1.1 Gear Preparation; For contruction of camera slides and mounts |
| 1/2" marine grade high density polyethylene | King Starboard | N/A | 1.1 Gear Preparation; For contruction of camera outer frame and camera mounting panels |
| 18-8 Flathead Stainless Steel Phillips Flat Head Screws, 3/8”-16 Thread Size, 1-1/2” Long | McMaster-Carr | 91771A628 | 1.1 Gear Preparation; For camera frame assembly |
| 18-8 Stainless Steel Socket Head Screw, 10-24 Thread Size, 1-1/8" Long | McMaster-Carr | 92196A248 | 1.1 Gear Preparation; Used to secure mounting plate to handles |
| 1 lb dive weights | House of Scuba | WBELT24 1LB | 1.2 Plot Setup; Used for reference floats |
| 200DL Underwater Housing for Nikon D780 DSLR Camera | Ikelite | 71019 | 1.1 Gear Preparation; Underwater housing for digital camera |
| 24mm fixed lens (AF-S NIKKOR 24mm f/1.8G ED) | Nikkor | 20057 | 1.1 Gear Preparation; Wide-angle lens for greater image overlap |
| 250# gray longline poly soft | Continental Western | 503086 | 1.2 Plot Setup; To build scale bars. Ground control markers for determining model scale |
| 3 lb drilling hammer | Estwing | B3-3LB | 1.2 Plot Setup; Used to install stainless steel stakes at survey site |
| 3/8-16 X 18" THRD ROD W/60 DEGREE POINT 316 S/S | Ababa Bolt | 37C1800ROD6/60DEG | 1.2 Plot Setup; For permanent installation to mark survey site |
| 316 Stainless Steel Nylon-Insert Locknut Super-Corrosion-Resistant, 1/4"-20 Thread Size | McMaster-Carr | 90715a125 | 1.2 Plot Setup; For scale bars and frame assembly. |
| 316 Stainless Steel Nylon-Insert Locknut Super-Corrosion-Resistant, 3/8"-16 Thread Size | McMaster-Carr | 90715A145 | 1.2 Plot Setup; Affixed to left stainless steel stake for orientation of the plot |
| 316 Stainless Steel Phillips Flat Head Screws, 10-32 Thread Size, 3/8" Long | McMaster-Carr | 91500a827 | 1.1 Gear Preparation; For camera frame assembly |
| 4"x4" Agisoft marker printed on waterproof paper | Agisoft | N/A | 1.2 Plot Setup; To build corner tiles. Ground control markers for determining model orientation |
| 4"x4"x1/4" Stainless steel tile | N/A | N/A | 1.2 Plot Setup; To build corner tiles. Ground control markers for determining model orientation |
| 4"x4"x3/4" custom printed plastic agisoft marker high density polyethylene color core | N/A | N/A | 1.2 Plot Setup; To build scale bars. Ground control markers for determining model scale |
| 512 GB Extreme PRO SDXC UHS-I Card - C10, U3, V30, 4K UHD, SD Card | SanDisk | SDSDXXY-512G-GN4IN | 1.1 Gear Preparation; High speed, large capacity storage card. Up to 2 used per camera for image storage |
| 5TB Elements Portable External Hard Drive HDD, USB 3.0 | Western Digital | WDBU6Y0050BBK-WESN | 1.3 Image Capture; Large volume external hard drive for image storage and image backup |
| 60 mm fixed lens (AF-S Micro NIKKOR 60mm F2.8G ED) | Nikkor | 2177 | 1.1 Gear Preparation; Macro zoom lens, optional for dual-camera setup |
| Acetal machined and tapped for 1"x12" 3/8" 16 thread support braces | N/A | N/A | 1.1 Gear Preparation; Camera frame support columns |
| AquaMend Epoxy Putty Stick | JD Industrial Supply | 470550 | 1.2 Plot Setup; Used to install stainless steel stakes at survey site |
| Architectural 6063 Aluminum U-Channel, 1/8" Wall Thickness, 1/2" High x 3/4" Wide Outside | McMaster-Carr | 9001k46 | 1.2 Plot Setup; To build scale bars. Ground control markers for determining model scale |
| Black-Oxide 18-8 Stainless Steel Pan Head Phillips Screws, 1/4"-20 Thread, 1/2" Long | McMaster-Carr | 91249a537 | 1.1 Gear Preparation; To attach ikelite handle to housing |
| Black-Oxide 18-8 Stainless Steel Pan Head Phillips Screws, 1/4"-20 Thread, 5/8" Long | McMaster-Carr | 91249A539 | 1.2 Plot Setup; To build scale bars. Ground control markers for determining model scale |
| Blue Steel Rope | Continental Western | 402020 | 1.2 Plot Setup; Used to secure dive float to the benthos during surveys |
| D780 camera body | Nikon | 1618 | 1.1 Gear Preparation; Camera body model |
| DGX Tech Compass w/Bungee Mount and Cord | Dive Gear Express | DX-9050x | 1.2 Plot Setup; For collection of plot bearings and as an addition to the camera frame as a navigational aid |
| Dive computer - Suunto Zoop Novo | Suunto | N/A | 1.2 Plot Setup; To record depth at reference tiles |
| Dive slate | TexWipe | TX5835 | 1.2 Plot Setup; Used to record plot metadata such as tile depth, and coded target numbers |
| DL 8 inch Dome Port | Ikelite | 75340 | 1.1 Gear Preparation; Dome port for underwater housing |
| FLOAT, PVC SPONGE, 5-3/4" DIA. BY 3/4", RUST | Memphis Net & Twine | SB1 | 1.2 Plot Setup; Used as a visual reference to determine plot boundaries and swim height of camera operator |
| Garmin 78s GPS | Garmin | 010-00864-01 | 1.2 Plot Setup; Used to record location of survey site |
| High performance computer | N/A | N/A | 2.0 Model Construction; For 3D dense point cloud processing, recommended specifications to include a high speed 10+ core CPU, 128GB RAM (64 GB minimum), 1TB solid state drive, and a dedicated NVIDIA or AMD GPU. |
| Inflatable surface dive float | Omer | Atol 6246 | 1.2 Plot Setup; Dual purpose surface marker buoy and |
| JOHNSON Cross Check Level: Nonmagnetic, 2 1/4 in x 1 7/16 in x 3/16 in, Plastic, Hanging Hole, 1mm/m | Grainger | 6C225 | 1.1 Gear Preparation; Optional addition to the camera frame as a navigational aid |
| Long Tape Measure,1/2 In x 30m,Pumpkin | Grainger | 3LJW9 | 1.2 Plot Setup; Used to set up plot area |
| Manta reel SR. Reel | Manta Industries | N/A | 1.2 Plot Setup; Attached to dive float for use during surveys |
| Metashape Professional License | Agisoft | N/A | 2.0 Model Construction; Software for dense point cloud processing |
| Non-glare clear acrylic | N/A | N/A | 1.2 Plot Setup; To build corner tiles. Ground control markers for determining model orientation |
| O-Ring 0132.45 for DL Port System, ULTRAcompact Housings | Ikelite | 132.45 | 1.1 Gear Preparation; O-ring for underwater housing |
| O-Ring 0132.59 for DSLR & Mirrorless Housings (Type 1) | Ikelite | 132.59 | 1.1 Gear Preparation; O-ring for underwater housing |
| Paracord or Dacron Line | N/A | N/A | 1.2 Plot Setup; Used to attach referene floats to dive weights |
| Passivated 18-8 Stainless Steel Phillips Flat Head Screw, 82 Degree Countersink, 1/4"-20 Thread, 1" Long | McMaster-Carr | 91771a542 | 1.1 Gear Preparation; Frame slider panel |
| Passivated 18-8 Stainless Steel Phillips Flat Head Screw, 82 Degree Countersink, 1/4"-20 Thread, 1-3/4" Long | McMaster-Carr | 91771a548 | 1.1 Gear Preparation; Frame slider panel |
| Passivated 18-8 Stainless Steel Phillips Flat Head Screw, 82 Degree Countersink, 10-32 Thread, 3/8" Long | McMaster-Carr | 91771A827 | 1.2 Plot Setup; To build corner tiles. Ground control markers for determining model orientation |
| Pelican 1060 micro case | Pelican | 1060-025-100 | 1.2 Plot Setup; Housing for GPS unit that is affixed to the inflatable dive float |
| Plastic-Head Thumb screw 1/4"-20 x 2" | McMaster-Carr | 91185A819 | 1.1 Gear Preparation; Use to secure camera housing to frame |
| Right Hand Quick Release Handle with Extension | Ikelite | 4077.02 | 1.1 Gear Preparation; Handle for underwater housing |
| R | N/A | N/A | 3.0 Ecological Analysis; Software for running structural complexity scripts |
| Taglab | N/A | N/A | 3.0 Ecological Analysis; Software for segmentation analysis |
| Trigger Extension v2 for Shutter or Back Button Focus | Ikelite | 4077.95 | 1.1 Gear Preparation; Trigger extenstion for underwater housing shutter button control |
| Vacuum pump with gauge | Ikelite | 47011 | 1.1 Gear Preparation; To test integrity of o-ring seals for underwater camera housings |
| Viscore | N/A | N/A | 2.0 Model Construction; 3.0 Ecological Analysis; Software for ecological analysis |
References
- Hubbell, S. P., Foster, R. B. Short-term dynamics of a neotropical forest: Why ecological research matters to tropical conservation and management. Oikos. 63, 48-61 (1992).
- Fahey, T. J., et al. The promise and peril of intensive-site-based ecological research: Insights from the Hubbard Brook ecosystem study. Ecology. 96 (4), 885-901 (2015).
- Condit, R., et al. Spatial patterns in the distribution of tropical tree species. Science. 288 (5470), 1414-1418 (2000).
- Lieberman, D., Lieberman, M., Peralta, R., Hartshorn, G. Mortality patterns and stand turnover rates in a wet tropical forest in Costa Rica. J Ecol. 73 (3), 915-924 (1985).
- Hubbell, S. P. Tree dispersion, abundance, and diversity in a tropical dry forest: That tropical trees are clumped, not spaced, alters conceptions of the organization and dynamics. Science. 203 (4387), 1299-1309 (1979).
- Connell, J. H. The consequences of variation in initial settlement vs. Post-settlement mortality in rocky intertidal communities. J Exp Mar Biol Ecol. 93 (1-2), 11-45 (1985).
- Turner, M. G. Landscape ecology: The effect of pattern on process. Annu Rev Ecol Syst. 20, 171-197 (1989).
- Rietkerk, M., Van De Koppel, J. Regular pattern formation in real ecosystems. Trends Ecol Evol. 23 (3), 169-175 (2008).
- Harms, K. E., Wright, S. J., Calderón, O., Hernandez, A., Herre, E. A. Pervasive density-dependent recruitment enhances seedling diversity in a tropical forest. Nature. 404 (6777), 493-495 (2000).
- Marhaver, K., Vermeij, M., Rohwer, F., Sandin, S. Janzen-connell effects in a broadcast-spawning caribbean coral: Distance-dependent survival of larvae and settlers. Ecology. 94 (1), 146-160 (2013).
- Kenyon, J. C., Maragos, J. E., Cooper, S. Characterization of coral communities at rose atoll, american samoa. Atoll Res Bull. 586, 1-28 (2010).
- Goreau, T. F. The ecology of jamaican coral reefs i. Species composition and zonation. Ecology. 40 (1), 67-90 (1959).
- Sandin, S. A., et al. Baselines and degradation of coral reefs in the northern line islands. PLoS One. 3 (2), e1548 (2008).
- Newman, M. J. H., Paredes, G. A., Sala, E., Jackson, J. B. C. Structure of Caribbean coral reef communities across a large gradient of fish biomass. Ecol Lett. 9 (11), 1216-1227 (2006).
- Smith, J. E., et al. Re-evaluating the health of coral reef communities: Baselines and evidence for human impacts across the central pacific. P Roy Soc B: Biol Sci. 283 (1822), 20151985 (2016).
- Lewis, J. B. Spatial distribution and pattern of some Atlantic reef corals. Nature. 227 (5263), 1158-1159 (1970).
- Bradbury, R. H., Young, P. C. The effects of a major forcing function, wave energy, on a coral reef ecosystem. Mar Ecol Prog Ser. 5, 229-241 (1981).
- Bak, R. P. M., Nieuwland, G. Long-term change in coral communities along depth gradients over leeward reefs in the Netherlands Antilles. Bull Mar Sci. 56 (2), 609-619 (1995).
- Connell, J. H., Hughes, T. P., Wallace, C. C. A 30-year study of coral abundance, recruitment, and disturbance at several scales in space and time. Ecol Monogr. 67 (4), 461-488 (1997).
- Hughes, T. P. Population dynamics based on individual size rather than age: A general model with a reef coral example. Am Nat. 123 (6), 778-795 (1984).
- Hughes, T. P., Tanner, J. E. Recruitment failure, life histories, and long-term decline in Caribbean corals. Ecology. 81 (8), 2250-2263 (2000).
- Fong, P., Glynn, P. A dynamic size-structured population model: Does disturbance control size structure of a population of the massive coral Gardineroseris planulata in the Eastern Pacific. Mar Biol. 130 (4), 663-674 (1998).
- Vardi, T., Williams, D. E., Sandin, S. A. Population dynamics of threatened elkhorn coral in the Northern Florida Keys, USA. Endanger Species Res. 19 (2), 157-169 (2012).
- Doropoulos, C., Ward, S., Roff, G., González-Rivero, M., Mumby, P. J. Linking demographic processes of juvenile corals to benthic recovery trajectories in two common reef habitats. PLoS One. 10 (5), e0128535 (2015).
- Edmunds, P. A quarter-century demographic analysis of the Caribbean coral, Orbicella annularis, and projections of population size over the next century. Limnol Oceanogr. 60 (3), 840-855 (2015).
- Deignan, L. K., Pawlik, J. R. Perilous proximity: Does the Janzen-Connell hypothesis explain the distribution of giant barrel sponges on a Florida coral reef. Coral Reefs. 34, 561-567 (2015).
- Zvuloni, A., et al. Spatio-temporal transmission patterns of black-band disease in a coral community. PLoS One. 4 (4), e4993 (2009).
- Karlson, R. H., Cornell, H. V., Hughes, T. P. Aggregation influences coral species richness at multiple spatial scales. Ecology. 88 (1), 170-177 (2007).
- Jolles, A. E., Sullivan, P., Alker, A. P., Harvell, C. D. Disease transmission of aspergillosis in sea fans: Inferring process from spatial pattern. Ecology. 83 (9), 2373-2378 (2002).
- Carlon, D. B., Olson, R. R. Larval dispersal distance as an explanation for adult spatial pattern in two Caribbean reef corals. J Exp Mar Biol Ecol. 173 (2), 247-263 (1993).
- Bak, R., Termaat, R., Dekker, R. Complexity of coral interactions: Influence of time, location of interaction and epifauna. Mar Biol. 69, 215-222 (1982).
- Stimson, J. An analysis of the pattern of dispersion of the hermatypic coral Pocillopora meandrina var. Nobilis verril. Ecology. 55 (2), 445-449 (1974).
- Edwards, C., et al. Large-area imaging in tropical shallow water coral reef monitoring, research and restoration: A practical guide to survey planning, execution, and data extraction. NOAA Technical Memorandum NOS NCCOS. (313), (2023).
- Pizarro, O., Eustice, R. M., Singh, H. Large area 3-d reconstructions from underwater optical surveys. IEEE J Oceanic Eng. 34 (2), 150-169 (2009).
- Figueira, W., et al. Accuracy and precision of habitat structural complexity metrics derived from underwater photogrammetry. Remote Sens. 7 (12), 16883-16900 (2015).
- Burns, J., Delparte, D., Gates, R., Takabayashi, M. Integrating structure-from-motion photogrammetry with geospatial software as a novel technique for quantifying 3d ecological characteristics of coral reefs. PeerJ. 3, e1077 (2015).
- Piazza, P., et al. Underwater photogrammetry in Antarctica: Long-term observations in benthic ecosystems and legacy data rescue. Polar Biol. 42, 1061-1079 (2019).
- Price, D. M., et al. Using 3d photogrammetry from rov video to quantify cold-water coral reef structural complexity and investigate its influence on biodiversity and community assemblage. Coral Reefs. 38, 1007-1021 (2019).
- Fallati, L., et al. Characterizing Håkon Mosby Mud Volcano (Barents Sea) cold seep systems by combining ROV-based acoustic data and underwater photogrammetry. Front Mar Sci. 10, 1269197 (2023).
- Ventura, D., et al. Seagrass restoration monitoring and shallow-water benthic habitat mapping through a photogrammetry-based protocol. J Environ Manage. 304, 114262 (2022).
- Combs, I. R., Studivan, M. S., Eckert, R. J., Voss, J. D. Quantifying impacts of stony coral tissue loss disease on corals in Southeast Florida through surveys and 3D photogrammetry. PLoS One. 16 (6), e0252593 (2021).
- Bongaerts, P., et al. Reefscape genomics: Leveraging advances in 3D imaging to assess fine-scale patterns of genomic variation on coral reefs. Front Mar Sci. 8, 638979 (2021).
- Raoult, V., Reid-Anderson, S., Ferri, A., Williamson, J. E. How reliable is Structure from Motion (sfm) over time and between observers? A case study using coral reef bommies. Remote Sens. 9 (7), 740 (2017).
- Weinberg, S. A comparison of coral reef survey methods. Bijdr Dierkd. 51 (2), 199-218 (1981).
- Edwards, C. B., et al. Large-area imaging reveals biologically driven non-random spatial patterns of corals at a remote reef. Coral Reefs. 36 (4), 1291-1305 (2017).
- Gracias, N., Santos-Victor, J. Underwater video mosaics as visual navigation maps. Comput Vis Image Und. 79 (1), 66-91 (2000).
- Lirman, D., et al. Development and application of a video-mosaic survey technology to document the status of coral reef communities. Environ Monit Assess. 125 (1-3), 59-73 (2007).
- Kodera, S. M., et al. Quantifying life history demographics of the scleractinian coral genus Pocillopora at Palmyra Atoll. Coral Reefs. 39 (4), 1091-1105 (2020).
- Ferrari, R., et al. 3D photogrammetry quantifies growth and external erosion of individual coral colonies and skeletons. Sci Rep. 7 (1), 16737 (2017).
- Sandin, S. A., et al. Considering the rates of growth in two taxa of coral across Pacific Islands. Adv Mar Biol. 87 (1), 167-191 (2020).
- Ventura, D., et al. Integration of close-range underwater photogrammetry with inspection and mesh processing software: A novel approach for quantifying ecological dynamics of temperate biogenic reefs. Remote Sens Ecol Conserv. 7 (2), 169-186 (2021).
- Ferrari, R., et al. Photogrammetry as a tool to improve ecosystem restoration. Trends Ecol Evol. 36 (12), 1093-1101 (2021).
- Remmers, T., et al. Close-range underwater photogrammetry for coral reef ecology: A systematic literature review. Coral Reefs. 43 (1), 35-52 (2024).
- Marre, G., Holon, F., Luque, S., Boissery, P., Deter, J. Monitoring marine habitats with photogrammetry: A cost-effective, accurate, precise and high-resolution reconstruction method. Front Mar Sci. 6, 276 (2019).
- Nocerino, E., et al. Comparison of diver-operated underwater photogrammetric systems for coral reef monitoring. Int Arch Photogramm Remote Sens Spat Inf Sci. 42 (2/W10), 143-150 (2019).
- Mccarthy, O. S., Smith, J. E., Petrovic, V., Sandin, S. A. Identifying the drivers of structural complexity on Hawaiian coral reefs. Mar Ecol Prog Ser. 702, 71-86 (2022).
- Pascoe, K. H., Fukunaga, A., Kosaki, R. K., Burns, J. H. 3D assessment of a coral reef at Lalo Atoll reveals varying responses of habitat metrics following a catastrophic hurricane. Sci Rep. 11 (1), 12050 (2021).
- Torres-Pulliza, D., et al. A geometric basis for surface habitat complexity and biodiversity. Nat Ecol Evol. 4 (11), 1495-1501 (2020).
- Friedman, A., Pizarro, O., Williams, S. B., Johnson-Roberson, M. Multi-scale measures of rugosity, slope and aspect from benthic stereo image reconstructions. PloS One. 7 (12), e50440 (2012).
- Hernández-Landa, R. C., Barrera-Falcon, E., Rioja-Nieto, R. Size-frequency distribution of coral assemblages in insular shallow reefs of the Mexican Caribbean using underwater photogrammetry. PeerJ. 8, e8957 (2020).
- Fukunaga, A., Burns, J. H., Pascoe, K. H., Kosaki, R. K. Associations between benthic cover and habitat complexity metrics obtained from 3D reconstruction of coral reefs at different resolutions. Remote Sens. 12 (6), 1011 (2020).
- Ferrari, R., et al. Quantifying multiscale habitat structural complexity: A cost-effective framework for underwater 3D modelling. Remote Sens. 8 (2), 113 (2016).
- Kopecky, K. L., et al. Quantifying the loss of coral from a bleaching event using underwater photogrammetry and ai-assisted image segmentation. Remote Sens. 15 (16), 4077 (2023).
- Sandin, S. A., et al. Evidence of biological self-organization in spatial patterns of a common tropical alga. Am Nat. 200 (5), 722-729 (2022).
- Burns, J. H. R., Alexandrov, T., Ovchinnikova, K., Gates, R. D., Takabayashi, M. Data for spatial analysis of growth anomaly lesions on Montipora capitata coral colonies using 3D reconstruction techniques. Data Br. 9, 460-462 (2016).
- Pedersen, N. E., et al. The influence of habitat and adults on the spatial distribution of juvenile corals. Ecography. 42, 1-11 (2019).
- Ferrari, R., et al. Quantifying the response of structural complexity and community composition to environmental change in marine communities. Glob Chang Biol. 22 (5), 1965-1975 (2016).
- Cresswell, A. K., et al. Structure-from-motion reveals coral growth is influenced by colony size and wave energy on the reef slope at Ningaloo Reef, Western Australia. J Exp Mar Biol Ecol. 530, 151438 (2020).
- Lange, I. D., Perry, C. T. A quick, easy and non-invasive method to quantify coral growth rates using photogrammetry and 3D model comparisons. Methods Ecol Evol. 11 (6), 714-726 (2020).
- George, E. E., et al. Space-filling and benthic competition on coral reefs. PeerJ. 9, e11213 (2021).
- Sarribouette, L., Pedersen, N. E., Edwards, C. B., Sandin, S. A. Post-settlement demographics of reef building corals suggest prolonged recruitment bottlenecks. Oecologia. 199 (2), 387-396 (2022).
- Lyons, M. B., et al. Mapping the world's coral reefs using a global multiscale earth observation framework. Remote Sens Ecol Conserv. 6 (4), 557-568 (2020).
- Ventura, D., et al. Coastal benthic habitat mapping and monitoring by integrating aerial and water surface low-cost drones. Front Mar Sci. 9, 1096594 (2023).
- Castellanos-Galindo, G. A., Casella, E., Mejía-Rentería, J. C., Rovere, A. Habitat mapping of remote coasts: Evaluating the usefulness of lightweight unmanned aerial vehicles for conservation and monitoring. Biol Conserv. 239, 108282 (2019).
- Fox, M. D., et al. Limited coral mortality following acute thermal stress and widespread bleaching on palmyra atoll, central pacific. Coral Reefs. 38, 701-712 (2019).
- Charendoff, J. A., et al. Variability in composition of parrotfish bite scars across space and over time on a central pacific atoll. Coral Reefs. 42 (4), 905-918 (2023).
- Petrovic, V., Vanoni, D. J., Richter, A. M., Levy, T. E., Kuester, F. Visualizing high resolution three-dimensional and two-dimensional data of cultural heritage sites. Mediterr Archaeol Ar. 20 (10), 93-100 (2014).
- Noaa Coral Program. . National coral reef monitoring plan. NOAA Coral Reef Conservation Program. , (2021).
- Goergen, E. A., et al. Coral reef restoration monitoring guide: Methods to evaluate restoration success from local to ecosystem scales. NOAA Technical Memorandum NOS NCCOS. 279, (2020).
- Pavoni, G., et al. Taglab: Ai-assisted annotation for the fast and accurate semantic segmentation of coral reef orthoimages. J Field Robot. 39 (3), 246-262 (2022).
- Coral Reef Ecosystem Program Pacific Islands Fisheries Science Center. . National coral reef monitoring program: Benthic complexity and urchin abundance at climate stations of the hawaiian archipelago since 2013. , (2016).
- Coral Reef Ecosystem Program Pacific Islands Fisheries Science Center. . National Coral Reef monitoring program: Benthic percent cover derived from analysis of benthic images collected for climate stations across the hawaiian archipelago since 2013. , (2021).
- Coral Reef Ecosystem Program Pacific Islands Fisheries Science Center. . National Coral Reef monitoring program: Stratified random surveys (strs) of coral demography (adult and juvenile corals) across the Hawaiian archipelago since 2013. , (2022).
- Couch, C. S., et al. Comparing coral colony surveys from in-water observations and structure-from-motion imagery shows low methodological bias. Front Mar Sci. 8, 647943 (2021).
- Barrera-Falcon, E., Rioja-Nieto, R., Hernández-Landa, R. C., Torres-Irineo, E. Comparison of standard Caribbean coral reef monitoring protocols and underwater digital photogrammetry to characterize hard coral species composition, abundance and cover. Front Mar Sci. 8, 722569 (2021).
- Carneiro, I. M., et al. Precision and accuracy of common coral reef sampling protocols revisited with photogrammetry. Mar Environ Res. 194, 106304 (2024).
- Curtis, J. S., Galvan, J. W., Primo, A., Osenberg, C. W., Stier, A. C. 3D photogrammetry improves measurement of growth and biodiversity patterns in branching corals. Coral Reefs. 42 (3), 623-627 (2023).
- Menna, F., Battisti, R., Nocerino, E., Remondino, F. Frog: A portable underwater mobile mapping system. Int Arch Photogramm Remote Sens Spat Inf Sci. 48, 295-302 (2023).
- Zhang, Y., Wang, Q., Shen, Y., He, B. An online path planning algorithm for autonomous marine geomorphological surveys based on AUV. Eng Appl Artif Intel. 118, 105548 (2023).
- Mccarthy, O. S., et al. Closing the gap between existing large-area imaging research and marine conservation needs. Conserv Biol. 38 (1), e14145 (2024).
- Pierce, J., Butler Iv, M. J., Rzhanov, Y., Lowell, K., Dijkstra, J. A. Classifying 3-D models of coral reefs using structure-from-motion and multi-view semantic segmentation. Front Mar Sci. 8, 706674 (2021).
- Runyan, H., et al. Automated 2D, 2.5 D, and 3D segmentation of coral reef pointclouds and orthoprojections. Front Robot AI. 9, 884317 (2022).
- Pavoni, G., Corsini, M., Pedersen, N., Petrovic, V., Cignoni, P. Challenges in the deep learning-based semantic segmentation of benthic communities from ortho-images. Appl Geomat. 13 (1), 131-146 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved