ポテンショスタット/Galvanostat を使用して担持触媒の電気化学測定
概要
ソース: 博士ユーリー ・ ローマンの研究所-マサチューセッツ工科大学
ポテンショスタット/galvanostat (単に電気化学測定装置とも呼ばれる) が応用の可能性 (定操作) で現在の措置または電位印加電流 (定電流動作) を測定する計測器 (図 1)。燃料電池、電解槽、電池、スーパーキャパシタの陽極および陰極材料の電気化学的特性評価で最もよく使用される楽器です。
従来、これらのアノードとカソード材料は 3 電極電気化学セルを介して電気化学測定装置と接続しました。電極リード線、ポテンシオスタットからは (興味の試験材料を含む) の作用電極、(補助電極とも呼ばれる) カウンター電極、参照電極に接続されます。電気化学セルは、酸性、アルカリ性、または塩のソリューションなどの高イオン強度電解質溶液で埋められます。この高いイオン強さの解決のためのメディアは通常水溶液です。ただし、用電池やスーパーキャパシタなどの潜在的な窓、セルを高め、動作を施行した非水溶液がよく使用されます。携帯メディアに脱気 (望ましくない副反応を防ぐ) ため不活性ガス、試験ガス (存在する場合は、テストの反応は、電極の一つの気体を含む)。
また、塩橋または膜を採用して場合、イオンの接触を維持するために 2 つ半分のセルに異なる電解質で測定されるあります。異種電極の作用電極でテスト分子も、対極の反応である場合、このタイプの「2 つのコンパートメント」細胞はよく使用されます。これは通常用いられる対極は多くの反応のための高活性触媒である白金と頻繁に起こる。ここでは、そのすべての 3 つの電極が同じメディアでは、単一コンパートメントのセルが使用されます。
このビデオは、作用電極の研磨、作用電極上の触媒インクを取り付け触媒インクを準備して、電気化学セルを準備する、電気化学測定を実行することのプロセスを説明します。実行される測定が含まれます: サイクリックボルタンメトリー (CV)、線形掃引ボルタンメトリー (LSV)、溶出クロノポテンショメトリー (CP)、クロノアンペロメトリー (CA)。
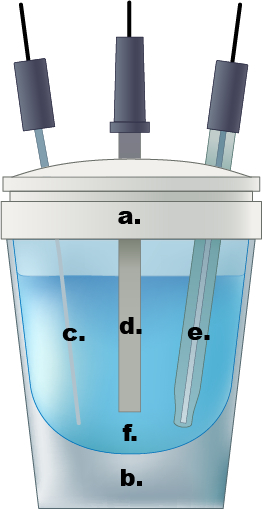
図 1。単一コンパートメント電気化学セルの例。a.) テフロン キャップ、b)ガラス細胞、c.)白金ワイヤ カウンター電極、d.)作用電極、e)Ag/AgCl 電極、f.)0.5 M 硫酸水溶液中の電解質溶液。
手順
1. 触媒インクと電極作製
安全上の注意:カーボン ブラックでサポートされている金属は、粉末吸入の危険性は、懸濁液の形ではそれまでヒューム フードやバランス筐体に扱われなければなりません。
- 囲まれたバランスを使用して、黒い金属/カーボン触媒の 5-10 mg を量りし、キャップをガラス瓶に追加します。
- 最終濃度が 1 mL の水に触媒の 7.5 mg になるよう、水と触媒を希釈ピペットを使用して。
- Sonicating 中、懸濁液 100 μ L の水の mL あたり Nafion 117 ソリューションが追加されます。
- インクは、均一分散を確保し、結合剤を混ぜてカーボン ブラック サポートの完全な少なくとも 10 分間超音波処理する必要があります。
- インクは sonicating 間 3 mm ガラス状カーボン ディスク電極のクリーンアップおよびソフト アルミナ パッドの旋回円を描くそれは 0.05 μ m のアルミナ溶液で覆われている摩擦によ
結果
申請書と概要
スキップ先...
このコレクションのビデオ:

Now Playing
ポテンショスタット/Galvanostat を使用して担持触媒の電気化学測定
Analytical Chemistry
51.8K 閲覧数

試料分析の準備のため
Analytical Chemistry
85.2K 閲覧数

社内基準
Analytical Chemistry
205.4K 閲覧数

標準添加法
Analytical Chemistry
320.7K 閲覧数

検量線
Analytical Chemistry
798.6K 閲覧数

(紫外-可視) 紫外可視分光法
Analytical Chemistry
625.2K 閲覧数

ラマン分光を用いた化学分析
Analytical Chemistry
51.4K 閲覧数

蛍光 x 線 (XRF)
Analytical Chemistry
25.9K 閲覧数

炎イオン化検出ガスクロマトグラフィー (GC)
Analytical Chemistry
283.0K 閲覧数

高速液体クロマトグラフィー (HPLC)
Analytical Chemistry
385.9K 閲覧数

イオン交換クロマトグラフィー
Analytical Chemistry
265.1K 閲覧数

キャピラリー電気泳動 (CE)
Analytical Chemistry
94.5K 閲覧数

質量分析への紹介
Analytical Chemistry
112.9K 閲覧数

走査型電子顕微鏡 (SEM)
Analytical Chemistry
87.6K 閲覧数

サイクリックボルタンメトリー (CV)
Analytical Chemistry
125.9K 閲覧数
Copyright © 2023 MyJoVE Corporation. All rights reserved