실온 이하의 반응 수행
Overview
출처: 다나 래슬리 박사연구소 - 윌리엄 대학교와 메리
데모: 맷 스미스
새로운 결합이 화학 반응의 과정에서 형성될 때, 관련 종 (원자 또는 분자)이 매우 근접하여 서로 충돌해야합니다. 이 종 사이 충돌은 이 분자가 움직이는 더 높은 속도 더 빈번하고 효과적입니다. Arrhenius 방정식1에뿌리를 두고 있는 널리 사용되는 엄지 손가락 규칙은 온도를 10K로 올리는 것이 반응 속도의 약 두 배가 되며 온도를 20K로 올리는 속도가 속도의 네 배가 될 것이라고 명시합니다.
(1) 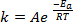
방정식 (1)은 종종 그 로그 와이트 형태로 발견된다 :
(2) 
여기서 K는 화학 반응의 속도이고, A는 주파수 인자(분자 충돌의 주파수에 관한),Ea는 반응에 필요한 활성화 에너지이며, R은 이상적인 가스 상수이며, T는 반응이 일어나는 온도이다.
따라서 온도가 높을수록 반응이 훨씬 더 빨리 완료된다는 것을 의미합니다. 그럼에도 불구하고, 어떤 경우에는 반응속도에 대한 저하 효과에도 불구하고 저온에서 반응을 수행하는 것이 바람직하다. 이 와 관련하여 몇 가지 시나리오는 아래에 자세히 설명되어 있습니다.
실온 보다 낮은 반응을 실행하는 것이 유용할 때, 화학자는 특정 온도 또는 온도 범위를 유지하기 위해 냉각 목욕을 사용합니다. 반응은 적절한 냉각 목욕 내부에 반응 플라스크를 배치하여 원하는 온도로 냉각됩니다. 반응의 시약은 냉각 목욕의 화학 물질과 직접 접촉하지 않습니다. 냉각 배스는 단일 극저온(냉각) 성분(예: 얼음, 드라이 아이스 또는 액체 질소)으로 구성될 수 있거나 특정 용매 및/또는 첨가제 염을 가진 극저온 성분의 혼합물일 수 있다. 용매의 목적은 냉각제의 온도를 효과적으로 반응 플라스크로 이송하는 것이며, 첨가제의 목적은 혼합물의 동결점을 낮게(또는 우울)하는 것이다. (물질이 용매와 첨가제일 수 있습니다.)
Procedure
쿨링 배스 설정
일반적인 설정의 경우, 아래에 설명된 대로 선택가능한 냉각 목욕을 준비하고 반응 플라스크를 욕조에 담급니다(도1). 목욕 용기를 끝까지 채우지 말고 반응 플라스크를 침수 할 수있는 충분한 공간을 남겨 둡니다.
참고: 반응이 수분에 민감한 경우 플라스크 또는 장치의 다른부분(예: 낙하 깔때기)에 시약을 추가할 때 매우 주의하십시오. 유리 제품이 냉각 욕조에 침지되는 동안 개구부가 생성되면 실온 공기가 빠르게 내부로 흐르고 수분을 운반합니다.

그림 1. 유입경로, 불활성 대기 하에서 온도계를 떨어뜨리는 3넥 라운드 하부 플라스크에 냉각 목욕 을 설정하는
Application and Summary
References
- Gordon, A. J., Ford, R. A. The Chemist's Companion - A Handbook of Practical Data, Techniques, and References. Chapter 11 (1973) ISBN: 978-0-471-31590-2.
- Rondeau, R. E. Slush baths. J. Chem. Eng. Data. 11, 124 (1966)
건너뛰기...
이 컬렉션의 비디오:

Now Playing
실온 이하의 반응 수행
Organic Chemistry
70.7K Views

촉매 소개
Organic Chemistry
34.5K Views

가열된 화학 반응을 위한 역류 시스템의 조립
Organic Chemistry
168.0K Views

솔벤트의 슐렌크 라인 전송
Organic Chemistry
41.7K Views

동결 펌프 해동 사이클링으로 액체 를 탈기
Organic Chemistry
56.3K Views

무수성 시약 및 장비 준비
Organic Chemistry
79.4K Views

재결정화로 화합물 정화
Organic Chemistry
709.6K Views

침전을 통한 혼합물의 분리
Organic Chemistry
157.9K Views

고체 액체 추출
Organic Chemistry
238.0K Views

용매제거를 위한 로타리 증발
Organic Chemistry
212.9K Views

분수 증류
Organic Chemistry
334.7K Views

X선 회절 분석을 위한 커지는 결정
Organic Chemistry
32.5K Views

Performing 1D Thin Layer Chromatography
Organic Chemistry
289.9K Views

열 크로마토그래피
Organic Chemistry
360.6K Views

핵 자기 공명 (NMR) 분광기
Organic Chemistry
248.6K Views
Copyright © 2025 MyJoVE Corporation. 판권 소유