A polyprotic acid contains more than one ionizable hydrogen and undergoes a stepwise ionization process. If the acid dissociation constants of the ionizable protons differ sufficiently from each other, then the titration curve for such polyprotic acid generates a distinct equivalence point for each of its ionizable hydrogens. Therefore, titration of a diprotic acid results in the formation of two equivalence points, whereas the titration of a triprotic acid results in the formation of three equivalence points on the titration curve.
Carbonic acid, H2CO3, is an example of a weak diprotic acid. The first ionization of carbonic acid yields hydronium ions and bicarbonate ions in small amounts.
First ionization:
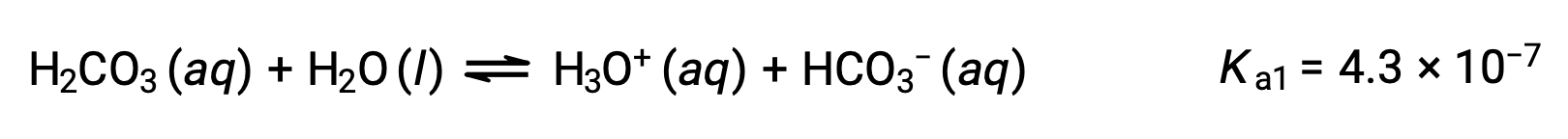
The bicarbonate ion can also act as an acid. It ionizes and forms hydronium ions and carbonate ions in even smaller quantities.
Second ionization:
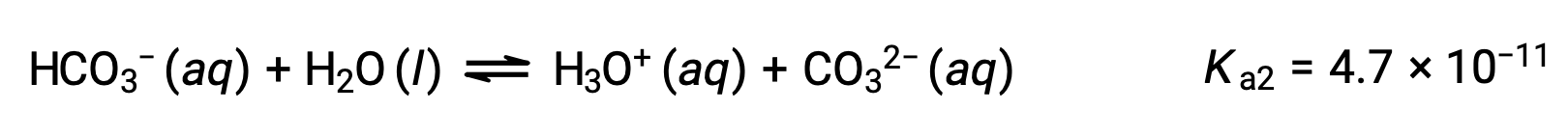
The Ka1 is larger than the Ka2 by a factor of 104. Therefore, when H2CO3 is titrated with a strong base like NaOH, it produces two distinct equivalence points for each ionizable hydrogen.
Phosphoric acid, a triprotic acid, ionizes in three steps:
First ionization:
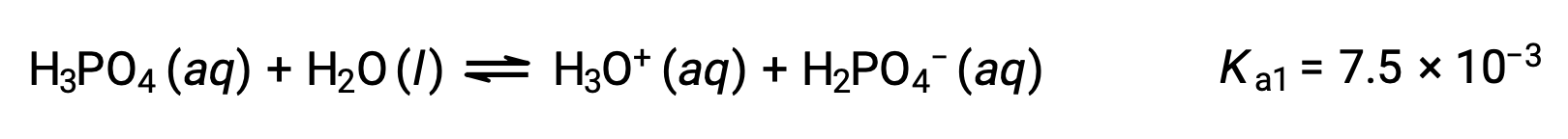
Second ionization:
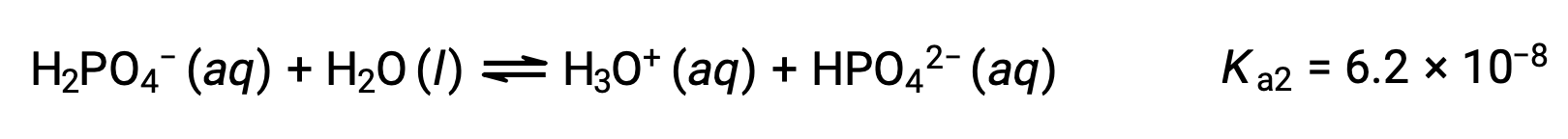
Third ionization:
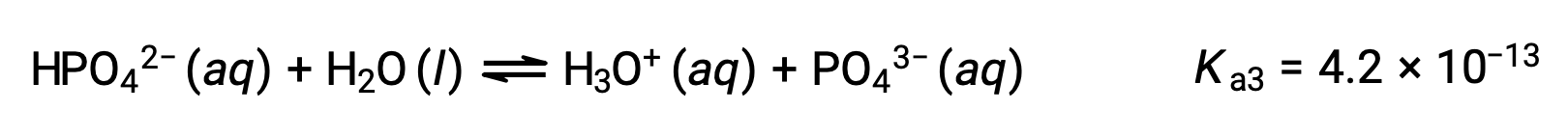
When H3PO4 is titrated with a strong base like KOH, it produces three equivalence points for each ionizable hydrogen. However, as HPO42− is a very weak acid, the third equivalence point is not easily discernible on the titration curve.
This text is adapted from Openstax, Chemistry 2e, Section 14.5: Polyprotic Acids.
Z rozdziału 16:

Now Playing
16.9 : Titration of a Polyprotic Acid
Acid-base and Solubility Equilibria
95.5K Wyświetleń

16.1 : Wspólny efekt jonowy
Acid-base and Solubility Equilibria
40.7K Wyświetleń

16.2 : Buffers
Acid-base and Solubility Equilibria
163.1K Wyświetleń

16.3 : Równanie Hendersona-Hasselbalcha
Acid-base and Solubility Equilibria
67.8K Wyświetleń

16.4 : Obliczanie zmian pH w roztworze buforowym
Acid-base and Solubility Equilibria
52.3K Wyświetleń

16.5 : Skuteczność bufora
Acid-base and Solubility Equilibria
48.3K Wyświetleń

16.6 : Obliczenia miareczkowania: mocny kwas - mocna zasada
Acid-base and Solubility Equilibria
28.8K Wyświetleń

16.7 : Obliczenia miareczkowania: słaby kwas - mocna zasada
Acid-base and Solubility Equilibria
43.6K Wyświetleń

16.8 : Wskaźniki
Acid-base and Solubility Equilibria
47.6K Wyświetleń

16.10 : Równowaga rozpuszczalności
Acid-base and Solubility Equilibria
51.5K Wyświetleń

16.11 : Czynniki wpływające na rozpuszczalność
Acid-base and Solubility Equilibria
32.9K Wyświetleń

16.12 : Powstawanie jonów złożonych
Acid-base and Solubility Equilibria
23.0K Wyświetleń

16.13 : Wytrącanie jonów
Acid-base and Solubility Equilibria
27.4K Wyświetleń

16.14 : Analiza jakościowa
Acid-base and Solubility Equilibria
19.9K Wyświetleń

16.15 : Krzywe miareczkowania kwasowo-zasadowego
Acid-base and Solubility Equilibria
125.9K Wyświetleń
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone