Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
A β-glucuronidase (GUS) Based Cell Death Assay
W tym Artykule
Podsumowanie
Programmed cell death assays commonly used in mammalian systems such as DNA laddering or TUNEL assays, are often difficult to reproduce in plants. In combination with a GUS reporter system, we propose a rapid, plant based transient assay to analyze the potential death properties of specific genes.
Streszczenie
We have developed a novel transient plant expression system that simultaneously expresses the reporter gene, β-glucuronidase (GUS), with putative positive or negative regulators of cell death. In this system, N. benthamiana leaves are co-infiltrated with a 35S driven expression cassette containing the gene to be analyzed, and the GUS vector pCAMBIA 2301 using Agrobacterium strain LBA4404 as a vehicle. Because live cells are required for GUS expression to occur, loss of GUS activity is expected when this marker gene is co-expressed with positive regulators of cell death. Equally, increased GUS activity is observed when anti-apoptotic genes are used compared to the vector control. As shown below, we have successfully used this system in our lab to analyze both pro- and anti-death players. These include the plant anti-apoptotic Bcl-2 Associated athanoGene (BAG) family, as well as, known mammalian inducers of cell death, such as BAX. Additionally, we have used this system to analyze the death function of specific truncations within proteins, which could provide clues on the possible post-translational modification/activation of these proteins. Here, we present a rapid and sensitive plant based method, as an initial step in investigating the death function of specific genes.
Protokół
Nicotiana benthamiana plants are grown in a temperature-controlled growth chamber at 25°C . Fully expanded healthy leaves of 3-6 week old plants are used.
Tip: Better results are obtained by using newly emerging leaves
1. Agrobacterium transient infiltration protocol:
Day 1
- Streak LB/Rifampicin (25 μg/ml)/Kanamycin (100 μg/ml) agar plates with glycerol stocks of Agrobacterium tumefaciens (strain LBA4404) containing the appropriate vectors with the gene (s) to be assayed for cell death and the vector containing the GUS cassette under a constitutive promoter. Always include an empty vector control as a negative control.
- Incubate at 28°C for 2 days.
Day 3
- Inoculate 2 ml of LB containing Rifampicin (25 μg/ml) and Kanamycin (100 μg/ml) with a single colony from each LB/Rifampicin/Kanamycin plate previously streaked.
- Incubate each culture by shaking at 28°C for 24 hours at 200 rpm until maximum growth density is reached.
Tip: Clumping is sometimes observed, in which case cultures need to be thoroughly resuspended (i.e. pipetting up and down) before proceeding.
Day 4
- Following incubation, increase the total volume to 10 mL with fresh LB (containing the appropriate antibiotics) and acetosyringone (final concentration 25 μM).
- Incubate each culture by shaking at 28 °C for another 16 hrs.
Day 5
- The following day wash each culture twice by adding 10 ml (do not add acetosyringone) infiltration medium (10 mM MgSO4.7H2O, 9 mM MES, pH 5.6) and centrifuge at 4000 x g for 10 min at room temperature.
- After the final centrifugation step, resuspend each culture in 5 ml infiltration medium containing acetosyringone (100 μM).
- Measure the OD600nm, measurements between 0.1 and 0.9 are acceptable.
Tip: OD600nm of as low as 0.1 have been used without a problem, however, higher ODs may produce more consistent results. - Incubate the cultures for 3 more hrs to prepare the Agrobacterium cultures for infection.
- Mix cultures containing the gene(s) to be analyzed and the negative control (Agrobacterium containing the empty vector) in a 1:1 ratio with the culture containing the GUS cassette.
- Infiltrate the abaxial (under) side of newly emerging leaves using a 1 ml needle-less syringe (Fig. 1).
Tip: Infiltrate both the negative control mixture (empty vector + GUS cassette) and the mixture containing the gene to be assayed (gene x + GUS cassette) on opposite leaves of the same plant. Use triplicate plants for each treatment. - 3 days post-infiltration excise infiltrated leaves and assay for GUS protein expression.
2. Histochemical GUS assay
Day 8
- Vacuum infiltrate the X-gluc substrate medium into the excised leaves.
- Incubate in darkness at room temperature overnight or until distinct blue staining appears.
Day 9
- Rinse in distillated water.
- Incubate in 70% ethanol until chlorophyll is removed, then transfer to distilled water again. Visually assess GUS expression levels.
3. Fluorometric MUG assay:
Day 8
- Isolate total protein by grinding infiltrated leaf discs in a mortar using liquid nitrogen. Add 100 uL of GUS extraction buffer (50 mM NaPi pH 7.0, 10 mM EDTA, 0.1% Triton X-100, 0.1 % N-lauroylsarcosine sodium salt, β-mercaptoethanol (0.7 μl/ml) while keeping the sample on ice.
- Centrifuge suspension at 14,000 g for 5 min at 4 °C and take supernatant as total soluble protein.
- Measure concentration by using a nanodrop according to manufacturer’s instructions.
- Adjust the protein concentration to 100 μg of total soluble protein for each sample using GUS extraction buffer.
- Add MUG substrate (4-methylumbelliferyl-β-D-glucuronide trihydrate) prepared in GUS extraction buffer to a final concentration of 2 mM. A total volume of 100 μL per sample (protein + MUG substrate) should be sufficient.
- Incubate the reactions for 1 hr at 37 °C.
- Stop reactions by adding 800 μl of GUS stop buffer (0.2 M Na2CO3).
- Measure fluorescence using a plate reader at an excitation wavelength of 365 nm and emission wavelength of 455 nm and compare values to an MU standard curve. Report levels of GUS activity as nmol MU / μg total soluble protein / min.
4. Stocks and Solutions:
- Acetosyringone (1 M) stock (FW = 196.2 g) – 0.981 g in 5 mL of DMSO
- Kanamycin (100 mg/ml) stock – prepare in dH20 – filter sterilize
- Rifampicin (25 mg/ml) stock – prepare in DMSO
- Luria-Bertani (LB) liquid growth media: (1L)
1 % (w/v) bacto-tryptone, 10 g
0.5 % (w/v) bacto-yeast extract, 5 g
170 mM sodium chloride 10 g
pH - 7 - Acetosyringone (25 μM) = 25 μl of a 1 M stock in a final volume of 1 ml
- Infiltration Medium: (1L)
10 mM MgSO4.7dH20(FW = 246.48 g), 2.4648 g
9 mM MES(FW = 213.25 g), 1.91925 g
pH – 5.6 - Acetosyringone (100 μM) = 100 μl of a 1M stock in a final volume of 1 ml
- GUS Extraction Buffer
50 mM NaPi pH 7.0, 10 mM EDTA, 0.1 % Triton X-100, 0.1 % N-lauroylsarcosine sodium salt, β-mercaptoethanol (0.7 μl/ml) - 2x Phosphate buffer
0.2M NaH2PO4 and 0.2 M Na2HPO4 pH 7 - X-Gluc substrate solution
Dissolve 1 mg of 5-bromo-4-chloro-3-indolyl b-D-glucuronide (X-Gluc) in 0.1 ml methanol. Add 1 ml 2x phosphate buffer, 20 μl 0.1M potassium ferricyanide, 10μl Triton X-100 10% and 850 μl of distillated water.
5. Representative results:
Following this protocol, loss of GUS expression is expected when cell death occurs. We have simultaneously expressed the reporter gene, β-glucuronidase (GUS) with a member of the cyto-protective Arabidopsis Bcl-2 Associated athanoGene (BAG) family. We co-infiltrated N. benthamiana leaves with a 35S driven BAG expression cassette and the GUS vector pCAMBIA 2301 using Agrobacterium strain LBA4404. As shown in figure 2, a visible increase in GUS staining was observed following this infiltration. Conversely, when the known pro-apoptotic member of the Bcl-2 family BAX was used, a marked reduction of GUS staining was observed (Fig. 2). In both of these cases, GUS expression was visibly different compared to the control. However, when the difference in expression is less evident, fluorometric MUG assays can be performed to quanitate GUS expression.

Figure 1. Example of Agrobacterium mixture infiltration of the abaxial side of N. benthamiana leaves using a 1 ml needle-less syringe.
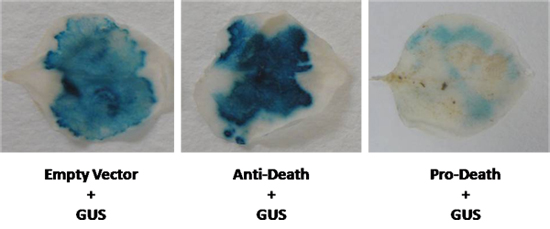
Figure 2. A GUS vector was co-expressed in N. benthamiana leaves with the anti-death gene, and a known inducer of cell death. GUS levels were compared to an empty vector control (GUS control).
Dyskusje
It is often difficult to use cell death detection techniques in plants that are common in mammalian systems. In combination with a GUS reporter system, we present a plant based, sensitive method for the detection and analysis of cell death players. This method takes advantage of the simple fact that live cells are required for GUS expression to occur. To ensure meaningful results and repeatability, it is critical that the cultures harboring the GUS cassette and the gene to be assayed are infiltrated at equal ratios. The...
Ujawnienia
No conflicts of interest declared.
Materiały
| Name | Company | Catalog Number | Comments |
| Name of the reagent | Company | Catalogue number | Comments (optional) |
|---|---|---|---|
| Rifampicin | VWR | IC19549001 | 25mg/mL stock in DMSO |
| 4'-hydroxy-3',5'-dimethoxyacetophenone (Acetosyringone) | VWR | TCD2666 | 0.981 g/mL in DMSO (1M stock) |
| 2-(4-morpholino)ethanesulfonic acid monohydrate (MES) | VWR | EM-6110 | 1.92 g/L in water for making 1 L of infiltration media |
| Bacto-tryptone | Fisher | BP1421-2 | 10g/L in water for making 1 L of LB medium |
| Bacto-yeast extract | VWR | EM1.03753.0500 | 5g/L in water for making 1 L of LB medium |
| Sodium chloride | VWR | EM-7710 | 10g/L in water for making 1 L of LB medium |
| Sodium phosphate monobasic monohydrate | VWR | MK-7868-12 | 2.5g/L in water for making 50mM of buffer NaPi |
| Sodium phosphate dibasic heptahydrate | VWR | EMD-SX0715-1 | 5g/L in water for making 50mM of buffer NaPi |
| Magnesium sulfate heptahydrate | VWR | EM-MX0070-1 | 2.5 g/L in water for making 1 L of infiltration media |
| N-Lauroylsarcosine | VWR | TCL0151-500G | 0.1% v/v in GUS buffer |
| 5-Bromo-4-chloro-3-indoxyl-beta-D-glucuronide cyclohexylammonium salt (X-gluc) | Gold Biotechnology | G1281C | 1mg/100uL in methanol 100% |
| 4-Methylumbelliferyl-μ-D-glucuronide hydrate (MUG) | Sigma | M5664 | 2 mM in 100 uL of GUS extraction buffer |
| Potassium ferrocyanide trihydrate | VWR | EM-PX1460-1 | 100mM stock for X-gluc substrate solution |
| β-Methylumbelliferone (MU) | Sigma-Aldrich | M1381 | For MU standard curve use GUS extraction buffer |
| Sodium carbonate | VWR | EM-SX0395-11 | 0.2 M in water for making GUS stop buffer |
Odniesienia
- Jefferson, R. A. GUS fusions: , β-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6, 3901-3901 (1987).
- Nishihara, M. Expression of the β-Glucuronidase Gene in Pollen of Lily (Lilium longiflorum), Tobacco (Nicotiana tabacum), Nicotiana rustica, and Peony (Paeonia lactiflora) by Particle Bombardment. Plant Physiol. 102, 357-357 (1993).
- Hodal, L. . Plant Science. 87, 115-115 (1992).
- Kabbage, M. The BAG proteins: a ubiquitous family of chaperone regulators. Cell Mol. Life Sci. . 65, 1390-1390 (2008).
- Kang, C. H. AtBAG6, a novel calmodulin-binding protein, induces programmed cell death in yeast and plants. Cell Death Differ. 13 (1), 84-84 (2006).
- Yan, J. . Plant Science. 165 (1), 1-1 (2006).
- Takayama, S., Reed, J. C. Molecular chaperone targeting and regulation by BAG family proteins. Nat Cell Biol. 3 (10), 237-237 (2001).
- Takayama, S. Cloning and functional analysis of BAG-1: a novel Bcl-2-binding protein with anti-cell death activity. Cell. 80 (2), 279-279 (1995).
- Vitha, S. Quantitative β-glucuronidase assay in transgenic plants. Biol. Plant. 35, 151-151 (1993).
- Otha, S. Construction and Expression in Tobacco of a β-Glucuronidase (GUS) Reporter Gene Containing an Intron Within the Coding Sequence. Plant Cell Physiol. 31, 805-805 (1990).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone