Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
A 3D System for Culturing Human Articular Chondrocytes in Synovial Fluid
W tym Artykule
Podsumowanie
A 3D system of culturing human articular chondrocytes in high levels of synovial fluid is described. Synovial fluid reflects the most natural microenvironment for articular cartilage, and can be easily obtained and stored. This system thus can be used for studying cartilage regeneration and for screening therapeutics for treating arthritis.
Streszczenie
Cartilage destruction is a central pathological feature of osteoarthritis, a leading cause of disability in the US. Cartilage in the adult does not regenerate very efficiently in vivo; and as a result, osteoarthritis leads to irreversible cartilage loss and is accompanied by chronic pain and immobility 1,2. Cartilage tissue engineering offers promising potential to regenerate and restore tissue function. This technology typically involves seeding chondrocytes into natural or synthetic scaffolds and culturing the resulting 3D construct in a balanced medium over a period of time with a goal of engineering a biochemically and biomechanically mature tissue that can be transplanted into a defect site in vivo 3-6. Achieving an optimal condition for chondrocyte growth and matrix deposition is essential for the success of cartilage tissue engineering.
In the native joint cavity, cartilage at the articular surface of the bone is bathed in synovial fluid. This clear and viscous fluid provides nutrients to the avascular articular cartilage and contains growth factors, cytokines and enzymes that are important for chondrocyte metabolism 7,8. Furthermore, synovial fluid facilitates low-friction movement between cartilaginous surfaces mainly through secreting two key components, hyaluronan and lubricin 9 10. In contrast, tissue engineered cartilage is most often cultured in artificial media. While these media are likely able to provide more defined conditions for studying chondrocyte metabolism, synovial fluid most accurately reflects the natural environment of which articular chondrocytes reside in.
Indeed, synovial fluid has the advantage of being easy to obtain and store, and can often be regularly replenished by the body. Several groups have supplemented the culture medium with synovial fluid in growing human, bovine, rabbit and dog chondrocytes, but mostly used only low levels of synovial fluid (below 20%) 11-25. While chicken, horse and human chondrocytes have been cultured in the medium with higher percentage of synovial fluid, these culture systems were two-dimensional 26-28. Here we present our method of culturing human articular chondrocytes in a 3D system with a high percentage of synovial fluid (up to 100%) over a period of 21 days. In doing so, we overcame a major hurdle presented by the high viscosity of the synovial fluid. This system provides the possibility of studying human chondrocytes in synovial fluid in a 3D setting, which can be further combined with two other important factors (oxygen tension and mechanical loading) 29,30 that constitute the natural environment for cartilage to mimic the natural milieu for cartilage growth. Furthermore, This system may also be used for assaying synovial fluid activity on chondrocytes and provide a platform for developing cartilage regeneration technologies and therapeutic options for arthritis.
Protokół
A 3D system for culturing human articular chondrocytes in synovial fluid
In this work, we encapsulated human articular chondrocytes in alginate beads using a modified manufacture-suggested encapsulation protocol (Lonza, and 31). Using these 3D constructs, we have developed a system for culturing cells in a culture medium containing varied percentages of human synovial fluid and have assessed these 3D constructs for cartilage gene expression.
1. Prepare human articular chondrocytes (HAC) for three-dimensional (3D) encapsulation
- Thaw a vial (1 ml) of human articular chondrocytes (HAC) (Lonza) (Passage 2) in 37°C water bath for 1 min.
- Mix with 1ml of chondrocyte growth media (Lonza) and centrifuge at 3000 rpm for 3-5 min to collect the cells. Discard the supernatant.
- Resuspend cell pellet in chondrocyte growth media (Lonza).
- Plate cells in a 10 cm tissue culture plate (BD Biosciences), and culture in chondrocyte growth media (Lonza) until the cells are confluent. HACs should be used at a passage number no higher than 3 or 4 (P3 or P4).
- Wash HACs on the plate with 155mM NaCl instead of the standard PBS, followed by trypsinzation to collect cells for alginate bead encapsulation. Once cells are detached, spin down the cells at 3000rpm for 5min. The cells are now ready for 3D encapsulation.
2. Encapsulate HACs into 3D beads
Resuspend the HACs in 1.2% alginate solution (Sigma) at a density of 8x105cells/ml. Cell numbers were determined prior to encapsulation, using a standard cell counter. It is very important to mix well to ensure even distribution of the cells in the beads.
- Pipette the HAC/Alginate solution mixture into a 12 ml syringe attached to a 22-gauge needle (Tyco healthcare, Inc.).
- Meanwhile, prepare a 50 mL beaker with 102 mM CaCl2 at a volume 5 times that of the HAC/aginate solution. Place a stir bar inside the beaker and stir the 102 mM CaCl2 solution slowly (at approximately 150 rpm).
- Hold the syringe tip about 6 inches above the surface of the CaCl2 solution and add the HAC/alginate mixture into the CaCl2 solution dropwise. In general, the resulting alginate beads are 2mm in diameter with an encapsulation density of approximately 104 cells/bead. Note: The height of the syringe over the CaCl2 solution is important. Shorter distance will result in tear-drop shaped instead of spherical shaped beads, causing uneven mechanical integrity and cell encapsulation.
- Let the beads stir in the CaCl2 solution for 20-25 min. While other alginate encapsulation protocols indicate a much shorter incubation time of 10min, we found that longer incubation time with the CaCl2 solution enhanced the integrity of the beads.
- Remove the CaCl2 solution, wash the beads 2-3 times in 2-3 volumes of NaCl, and then once in chondrocyte differentiation medium (Lonza). While other alginate bead encapsulation protocols involve using a filter for the above washing procedures, we found that the alginate beads often become trapped in the filter and dry out, thus reducing the efficiency of encapsulation. We found it most efficient to allow the beads to settle to the bottom of the beaker before discarding the solution.
- Use a standard spatula to transfer the beads into culture dishes. We usually culture 12 beads per 3 cm well for 2 days in chondrocyte growth medium to allow chondrocytes to adjust to the encapsulation process in the medium they were grown in before switching to chondrocyte differentiation medium (Lonza) with synovial fluid.
3. Culture chondrocytes in synovial fluid culture medium
- Fresh synovial fluid can be obtained from an outpatient clinic (We obtained synovial fluid from Tufts Medical Center). The synovial fluid can be transferred in 15ml falcon tubes to the laboratory, and immediately centrifuged at 3000 rpm for 15min to remove cell debris. Aliquot cell-free synovial fluid into 1.5 mL microcentrifuge tubes, to avoid repeated freeze-thawing. Supernatant can be stored at -80°C until use.
- Prior to culturing, mix the synovial fluid with chondrocyte differentiation medium (CDM, Lonza) at varying volumetric ratios, with a constant concentration of 100mM ascorbic acid and 9 mM CaCl2 (this trace amount of CaCl2 is to ensure the integrity of the alginate beads).
- Keep the plate on a rocking platform in a 37°C incubator (rocking frequency: about 75 times/min) to help nutrient distribution within the alginate beads, and to reduce clumping of the beads. This is especially important as we culture these chondrocytes for an extended period of time (up to 4 weeks). Note: While chondrocyte growth and differentiation media (Lonza) contain two commonly used antibiotics gentamycin and amphotericin, we did not supplement any antibiotics when we used different percentage of synovial fluid for chondrocyte culturing. No contamination was ever observed.
- Change media mixtures every 2-3 days. The viscosity of synovial fluid has been a major hurdle in culturing chondrocytes encapsulated in alginate beads during long-term cultures. We found it most effective to dilute synovial fluid-supplemented medium with 102mM CaCl2 (50% V/V), which also strengthens the integrity of the alginate beads. In this way, the medium can slowly be removed with a pipet, without damaging the beads.
4. Harvest HACs in alginate beads for gene expression analysis
Special care must be taken when harvesting the HACs from the alginate beads for gene expression analysis.
- Wash the alginate beads 3 times in 102 mM CaCl2 for about 5 min each time.
- Retrieve the chondrocytes by adding 55 mM NaCitrate at a volume 4-5 times that of the beads. It is important that the alginate beads are totally immersed in the NaCitrate solution. Shake or rock for 20-30 min.
- Spin down the cells and discard the supernatant.
- For RT-PCR analysis, resuspend the cell pellet in cell lysis buffer (Qiagen RNA isolation kit), and proceed with RNA purification.
5. Fix HACs in alginate beads for histological analysis
Special care has to be taken to harvest the HACs from the 3D beads for histological analysis.
- Wash the alginate beads 3 times in 102 mM CaCl2 for about 5 min each time. To completely wash away the viscous synovial fluid, we found it most effective to wash with chondrocyte differentiation medium (Lonza) overnight, rocking in a 37°C incubator (rocking frequency: about 75 times/min). The chondrocyte growth medium was used to ensure chondrocyte survival in this prolonged wash and to allow maximum penetration of the fixing agent.
- Fix the beads in 70% ethanol overnight, and proceed with histological analysis. To perform Dapi staining, incubate alginate beads with Dapi solution (500ng/ml) for 1hr under gentle rocking, and wash for 3 times with 102mM CaCl2 for 15 min each time. Dapi images can then be viewed under a fluorescent microscope. The beads can also be sectioned for alcian blue and H&E staining.
6. Representative Results
Our 3D culturing method for human chondrocytes in high percentages of synovial fluid is depicted in the schematic diagram shown in Fig.1. After human chondrocytes were encapsulated in alginate beads, they were allowed to grow in medium supplemented with varying ratios of synovial fluid. Because of the viscosity of the synovial fluid, it is essential to culture chondrocytes under constant rocking conditions to prevent the clumping of the cartilage constructs and to ensure even distribution of the nutrients. It is also essential to wash the alginate beads extensively before retrieving the chondrocytes, so that the fixing or lysis buffer can penetrate the beads (Fig.1). Bright field (BF) images of chondrocytes in the alginate beads are shown in Fig.2. Dapi staining was performed to confirm usniform distribution of the cells within the beads (Fig. 2). At the end of the 21-day culture period, gene expression analysis was performed by qRT-PCR. The reference gene GAPDH was used for normalization for all PCRs, as it was determined to be one of the most reliable reference genes for qPCR analysis on chondrocytes 32. An example is shown in Fig. 3, where we analyzed the results from human articular chondrocytes cultured in synovial fluid pooled from six patients with osteoarthritis. Consistent with the fact that the chondrocytes from Lonza have been expanded in 2D cultures, which would inevitably lead to de-differentiation 33, we found that Day 0 chondrocytes expressed minimal levels of cartilage matrix genes (Fig. 3). 3D culturing of chondrocytes with chondrocyte differentiation medium (Lonza) or medium supplemented with synovial fluid significantly increased cartilage gene expression of collagen, aggrecan and MMP13, which indicates chondrocyte re-differentiation by Day 21 (Fig.3) 34. Increasing the percentage of synovial fluid in the media resulted in comparable levels of cartilage matrix markers collagen II and aggrecan mRNA expression (Fig.3A and 3B). Furthermore, chondrocytes cultured in 100% synovial fluid even exhibited a decrease in cartilage degrading enzyme MMP13 mRNA expression as compared with those cultured in medium alone (Fig.3C). Interestingly, the expression level of cell death indicator caspase 3 gradually decreased with increasing ratios of synovial fluid, suggesting that synovial fluid culturing has led to decreased apoptosis levels (Fig. 3D). Therefore, our results show that culturing human articular chondrocytes in high levels of synovial fluid in a 3D setting is a feasible technology.
Tables and Figures

Figure 1. Schematic diagram of the method to culture human articular chondrocytes in high percentages of synovial fluid in 3D alginate beads. First, chondrocytes and alginate solution are mixed. When applied drop-wise to the CaCl2 solution, chondrocytes are immobilized within the crosslinked Ca-alginate hydrogel beads. These 3D constructs are then cultured in the chondrocyte differentiation medium (Lonza) with varying ratios of human synovial fluid. After 21 days of culturing under a rocking condition, alginate beads containing cells are washed extensively after which the cells are retrieved for gene expression analysis.
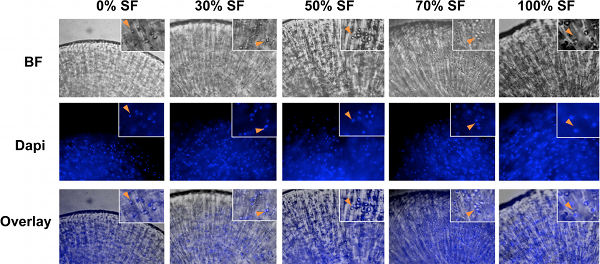
Figure 2. Bright field (BF) and Dapi images of human articular chondrocytes encapsulated cultures with 0%, 30%, 50%, 70% and 100% synovial fluid. Chondrocytes were spherical in shape in all culture conditions. Images of bright field (BF) and Dapi were overlaid to confirm the location and distribution of chondrocytes. Insets, magnified images. Arrowheads, colocalization of chondrocytes in BF and Dapi staining images.
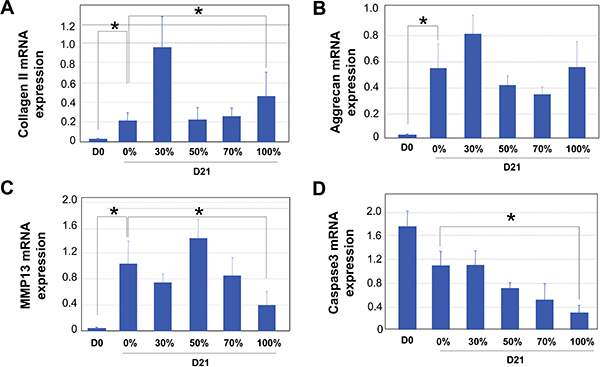
Figure 3. qRT-PCR analysis of encapsulated human articular chondrocytes at day 0 (D0) an after 21 days of culturing (D21) in medium supplemented with varying ratios of synovial fluid (SF) (0%, 30%, 50%, 70% and 100%). Results of four independent samples are shown here. GAPDH was used as internal reference for all PCRs. A. Collagen II mRNA expression. B. Aggrecan mRNA expression. C. MMP13 mRNA expression. D. Caspase 3 mRNA expression. Statistical significance was assessed for Day 0 samples and Day 21 samples of 0% and 100% synovial fluid (SF) culturing, using INSTAT software. * denotes P<0.05.
Dyskusje
In this report, we developed a method that allows for the culture of human articular chondrocytes in a 3D environment in medium that contains high concentrations of human synovial fluid. Synovial fluid is one of the major components that constitute the natural environment in the joint cavity, where articular chondrocytes reside. However, the viscosity of the synovial fluid has been a major challenge for three-dimensional long-term culturing of chondrocytes. To overcome the challenge of maintaining even nutrient distribut...
Ujawnienia
We have nothing to disclose.
Podziękowania
We would like to thank Robin Nye (Tufts Medical Center), Tomoya Uchimura and Dana Cairns (Tufts University) for providing help with synovial fluid storage and centrifugation. This work was funded by the NIH (1R01AR059106-01A1) for L.Z.
Materiały
| Name | Company | Catalog Number | Comments |
| Table of specific reagents and equipment: | |||
| Name of the Reagent | Company | Catalogue Number | Comments |
| Alginate (Alginic Acid sodium salt) | Sigma-Aldrich | A2158-250G | 2.4% solution stored at 40°C |
| Calcium Chloride Dihydrate, Granular | JT Baker | A19339 | |
| Chondrogenic Growth media | Lonza Inc. | CC-3156 (base media) | |
| CC-4409 (supplement) | |||
| Chondrogenic Differentiation Media | Lonza Inc. | CC-3226 (base media) | |
| CC-4408 (supplement) | |||
| Human articular chondrocytes | Lonza Inc. | CC-2550 | |
| Dapi (4′,6-Diamidino-2-phenylindole dihydrochloride) | Sigma-Aldrich | D9542 | |
| RNeasy mini kit (for RNA extraction) | Qiagen | 74104 | |
| PCR reagents: SYBR-green | Quanta Biosciences | 95053-500 | |
| 12 ml syringe | Tyco Healthcare, Covidien | 512852 | |
| 22-Gague Hypodermic Needle | Tyco Healthcare, Covidien | 8881 | |
| Microscope | Olympus Corporation | IX71 | |
| Platform rocker | Thermo Fisher Scientific, Inc. | Vari-mix | |
| Collagen IIa-forward | 5’-TTC ATC CCA CCC TCT CAC AGT-3’ | ||
| Collagen IIa-reverse | 5’-CCTCTGCCTTGACCCGAA-3’ | ||
| MMP13-forward | 5’-TGT GCC CTT CTT CAC ACA GAC ACT-3’ | ||
| MMP13-reverse | 5’-GAG AGC AGA CTT TGA GTC ATT GCC-3’ | ||
| Caspase 3-forward | 5’-TCA TTA TTC AGG CCT GCC GTG GTA-3’ | ||
| Caspase 3-reverse | 5’-TGG ATG AAC CAG GAG CCA TCC TTT -3’ | ||
Odniesienia
- Centers for Disease Control and P. Projected state-specific increases in self-reported doctor-diagnosed arthritis and arthritis-attributable activity limitations--United States, 2005-2030. MMWR. Morb. Mortal. Wkly. Rep. 56, 423-425 (2007).
- Theis, K. A., Murphy, L., Hootman, J. M., Helmick, C. G., Yelin, E. Prevalence and correlates of arthritis-attributable work limitation in the US population among persons ages 18-64: 2002 National Health Interview Survey Data. Arthritis Rheum. 57, 355-363 (2007).
- Chung, C., Burdick, J. A. Engineering cartilage tissue. Adv. Drug. Deliv. Rev. 60, 243-262 (2008).
- Glowacki, J. In vitro engineering of cartilage. J. Rehabil. Res. Dev. 37, 171-177 (2000).
- Chokalingam, K., Hunter, S. A., Gooch, C. 3D-In vitro Effects of Compression and Time in Culture on Aggregate Modulus and on Gene Expression and Protein content of Collagen Type II in Murine Chondrocytes. Tissue Eng. Part A. , (2009).
- Butler, D. L., Goldstein, S. A., Guilak, F. Functional tissue engineering: the role of biomechanics. J. Biomech. Eng. 122, 570-575 (2000).
- Goldring, M. B., Goldring, S. R. Osteoarthritis. J. Cell. Physiol. 213, 626-634 (2007).
- Zvaifler, N. J., Firestein, G. S. Cytokines in chronic inflammatory synovitis. Scand. J. Rheumatol. Suppl. 76, 203-210 (1988).
- Rhee, D. K., Marcelino, J., Baker, M. The secreted glycoprotein lubricin protects cartilage surfaces and inhibits synovial cell overgrowth. J. Clin. Invest. 115, 622-631 (2005).
- Campo, G. M., Avenoso, A., Nastasi, G. Hyaluronan reduces inflammation in experimental arthritis by modulating TLR-2 and TLR-4 cartilage expression. Biochim. Biophys. Acta. , (2011).
- van de Lest, C. H., van den Hoogen, B. M., van Weeren, P. R. Loading-induced changes in synovial fluid affect cartilage metabolism. Biorheology. 37, 45-55 (2000).
- Saxne, T., Heinegard, D., Wollheim, F. A. Human arthritic synovial fluid influences proteoglycan biosynthesis and degradation in organ culture of bovine nasal cartilage. Coll. Relat. Res. 8, 233-247 (1988).
- Lee, D. A., Salih, V., Stockton, E. F., Stanton, J. S., Bentley, G. Effect of normal synovial fluid on the metabolism of articular chondrocytes in vitro. Clin. Orthop. Relat. Res. , 228-238 (1997).
- Schalkwijk, J., Joosten, L. A., van den Berg, W. B., van de Putte, L. B. Chondrocyte nonresponsiveness to insulin-like growth factor 1 in experimental arthritis. Arthritis Rheum. 32, 894-900 (1989).
- Schalkwijk, J., Joosten, L. A., van den Berg, W. B., van Wyk, J. J., van de Putte, L. B. Insulin-like growth factor stimulation of chondrocyte proteoglycan synthesis by human synovial fluid. Arthritis Rheum. 32, 66-71 (1989).
- Joosten, L. A., Schalkwijk, J., van den Berg, W. B., van de Putte, L. B. Chondrocyte unresponsiveness to insulin-like growth factor-1. A novel pathogenetic mechanisms for cartilage destruction in experimental arthritis. Agents Actions. 26, 193-195 (1989).
- Schuerwegh, A. J., Dombrecht, E. J., Stevens, W. J. Synovial fluid and peripheral blood immune complexes of patients with rheumatoid arthritis induce apoptosis in cytokine-activated chondrocytes. Rheumatol. Int. 27, 901-909 (2007).
- Hegewald, A. A., Ringe, J., Bartel, J. Hyaluronic acid and autologous synovial fluid induce chondrogenic differentiation of equine mesenchymal stem cells: a preliminary study. Tissue Cell. 36, 431-438 (2004).
- Xu, Q. R., Dong, Y. H., Chen, S. L., Bao, C. D., Du, H. Degeneration of normal articular cartilage induced by late phase osteoarthritic synovial fluid in beagle dogs. Tissue Cell. 41, 13-22 (2009).
- Kruger, J. P., Endres, M., Neumann, K., Haupl, T., Erggelet, C., Kaps, C. Chondrogenic differentiation of human subchondral progenitor cells is impaired by rheumatoid arthritis synovial fluid. J. Orthop. Res. 28, 819-827 (2010).
- Steinhagen, J., Bruns, J., Niggemeyer, O. Perfusion culture system: Synovial fibroblasts modulate articular chondrocyte matrix synthesis in vitro. Tissue Cell. 42, 151-157 (2010).
- Yang, K. G., Saris, D. B., Verbout, A. J., Creemers, L. B., Dhert, W. J. The effect of synovial fluid from injured knee joints on in vitro chondrogenesis. Tissue Eng. 12, 2957-2964 (2006).
- Skoog, V., Widenfalk, B., Ohlsen, L., Wasteson, A. The effect of growth factors and synovial fluid on chondrogenesis in perichondrium. Scand. J. Plast. Reconstr. Surg. Hand. Surg.. 24, 89-95 (1990).
- Nuver-Zwart, I., Schalkwijk, J., Joosten, L. A., van den Berg, W. B., van de Putte, L. B. Effects of synovial fluid and synovial fluid cells on chondrocyte metabolism in short term tissue culture. J. Rheumatol. 15, 210-216 (1988).
- Beekhuizen, M., Bastiaansen-Jenniskens, Y. M., Koevoet, W. Osteoarthritic synovial tissue inhibits proteoglycan production in human osteoarthritic cartilage; Establishment and characterisation of a long-term coculture. Arthritis Rheum. , (2011).
- Rodrigo, J. J., Steadman, J. R., Syftestad, G., Benton, H., Silliman, J. Effects of human knee synovial fluid on chondrogenesis in vitro. Am. J. Knee. Surg. 8, 124-129 (1995).
- van den Hoogen, B. M., van de Lest, C. H., van Weeren, P. R. Loading-induced changes in synovial fluid affect cartilage metabolism. Br. J. Rheumatol. 37, 671-676 (1998).
- Webb, G. R., Westacott, C. I., Elson, C. J. Osteoarthritic synovial fluid and synovium supernatants up-regulate tumor necrosis factor receptors on human articular chondrocytes. Osteoarthritis Cartilage. 6, 167-176 (1998).
- Kook, S. H., Son, Y. O., Lee, K. Y. Hypoxia affects positively the proliferation of bovine satellite cells and their myogenic differentiation through up-regulation of MyoD. Cell. Biol. Int. 32, 871-878 (2008).
- Knobloch, T. J., Madhavan, S., Nam, J., Agarwal, S., Agarwal, S. Regulation of chondrocytic gene expression by biomechanical signals. Crit. Rev. Eukaryot. Gene. Expr. 18, 139-150 (2008).
- Guo, J. F., Jourdian, G. W., MacCallum, D. K. Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Connect. Tissue. Res. 19, 277-297 (1989).
- Toegel, S., Huang, W., Piana, C. Selection of reliable reference genes for qPCR studies on chondroprotective action. BMC. Mol. Biol. 8, 13-13 (2007).
- Lin, Z., Fitzgerald, J. B., Xu, J. Gene expression profiles of human chondrocytes during passaged monolayer cultivation. J. Orthop. Res. 26, 1230-1237 (2008).
- Goessler, U. R., Bieback, K., Bugert, P. Human chondrocytes differentially express matrix modulators during in vitro expansion for tissue engineering. Int. J. Mol. Med. 16, 509-515 (2005).
- Anat, J. . 121, 107-118 (1976).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone