Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Modeling Intracerebral Hemorrhage in Mice: Injection of Autologous Blood or Bacterial Collagenase
W tym Artykule
Podsumowanie
Clinically relevant animal models of intracerebral hemorrhage (ICH) are needed to extend our knowledge of hemorrhagic stroke and to examine novel therapeutic strategies. In this study, we describe and evaluate two ICH models that implement unilateral injections of either autologous whole blood or bacterial collagenase into the basal ganglia (corpus striatum) of mice.
Streszczenie
Spontaneous intracerebral hemorrhage (ICH) defines a potentially life-threatening neurological malady that accounts for 10-15% of all stroke-related hospitalizations and for which no effective treatments are available to date1,2. Because of the heterogeneity of ICH in humans, various preclinical models are needed to thoroughly explore prospective therapeutic strategies3. Experimental ICH is commonly induced in rodents by intraparenchymal injection of either autologous blood or bacterial collagenase4. The appropriate model is selected based on the pathophysiology of hemorrhage induction and injury progression. The blood injection model mimics a rapidly progressing hemorrhage. Alternatively, bacterial collagenase enzymatically disrupts the basal lamina of brain capillaries, causing an active bleed that generally evolves over several hours5. Resultant perihematomal edema and neurofunctional deficits can be quantified from both models. In this study, we described and evaluated a modified double injection model of autologous whole blood6 as well as an ICH injection model of bacterial collagenase7, both of which target the basal ganglia (corpus striatum) of male CD-1 mice. We assessed neurofunctional deficits and brain edema at 24 and 72 hr after ICH induction. Intrastriatal injection of autologous blood (30 μl) or bacterial collagenase (0.075U) caused reproducible neurofunctional deficits in mice and significantly increased brain edema at 24 and 72 hr after surgery (p<0.05). In conclusion, both models yield consistent hemorrhagic infarcts and represent basic methods for preclinical ICH research.
Protokół
All procedures were conducted in compliance with the NIH Guide for the Care and Use of Laboratory Animals and approved by the Animal Care and Use Committee at Loma Linda University.
1. Presurgical Preparations
Aseptic techniques are recommended for all surgical procedures. Disinfect the stereotactic apparatus and prepare sterile surgical tools prior to surgery. Wear personal protective equipment (PPE) during all animal handling. Use a heating pad during surgery to maintain the animal's physiologic body temperature.
- Weigh the 8-12 week old mouse using a triple beam animal scale.
- Co-inject ketamine (100 mg/kg) and xylazine (10 mg/kg) intraperitoneally then allow 7-10 min for the anesthesia to take effect (monitor for an adequate sedation).
- Place the mouse onto a thermal blanket and shave the scalp.
- Apply ophthalmic ointment to both eyes.
- Secure the airway, by gently moving the tongue laterally, and carefully secure the mouse's head onto the stereotactic apparatus. Note: The head must be secured horizontally to the basis of the stereotactic frame.
- Disinfect the surgical area with Betadine, and rinse with 70% ethanol. Repeat alternating applications of Betadine and 70% ethanol for a total of three times. Cotton-tipped applicators can be used for this purpose.
2. Blood Injection Model
- Make a 1 cm long midline incision of the scalp with a #10 scalpel blade.
- Use cotton-tipped applicators to clear away the soft tissue covering the skull, in order to expose the perpendicular intersection point of the coronal and sagittal suture (bregma).
- Mount the Hamilton syringe (250 μl) onto the injection pump, and stereotaxically direct the needle (26 Gauge) over bregma.
- Next, adjust the stereotactic manipulator arms to position the needle 0.2 mm anterior and 2 mm laterally to the right. At these coordinates make a small cranial burr hole, using a variable speed drill with a 1 mm drill bit.
- Suspend the animal's tail and disinfect its lower surface with 70% ethanol.
- Puncture the central tail artery with a sterile needle (e.g. 26 Gauge) and collect the arterial blood into an unheparinized capillary tube.
- Transfer the blood quickly from the capillary tube into the glass barrel of the Hamilton syringe, then insert the plunger.
- Reattach the now 30 μl or more of arterial blood containing Hamilton syringe onto the injection pump and insert the needle (with its beveled edge facing the sagittal suture) through the burr hole just until its bevel is no longer visible.
- From this point advance the needle 3 mm ventrally and inject 5 μl of autologous blood at a rate of 2 μl/min.
- After completion of the first injection advance the needle 0.7 mm further in depth.
- Wait for 5 min then inject 25 μl of blood into the right striatum.
- Upon completion of the second injection, leave the needle in position for additional 10 min, before withdrawing it at a rate of 1 mm/min.
- Seal the burr hole with bone wax and suture the skin.
- For postoperative analgesia inject 0.05 mg/kg of buprenorphine subcutaneously in pre-warmed fluids (normal saline).
3. Collagenase Injection Model
- Following the presurgical preparations, repeat steps 1-4 as described for the blood injection model.
- Fill the Hamilton syringe (10 μl) with 0.075U of bacterial (clostridial) collagenase VII-S dissolved in 0.5 μl of saline. Avoid the formation of air bubbles.
- Reattach the Hamilton syringe onto the injection pump and insert the needle (26 Gauge), through the burr hole just until its bevel is no longer visible.
- Advance the needle 3.7 mm ventrally and inject the 0.075U of collagenase into the right striatum at a rate of 2 μl/min.
- Upon completion of the injection, leave the needle in position for additional 10 min, before withdrawing it at a rate of 1 mm/min.
- Seal the burr hole with bone wax and suture the skin.
- Inject 0.05 mg/kg of buprenorphine subcutaneously in pre-warmed post-operative fluids.
4. Sham Operation
- Following the presurgical preparations, repeat steps 1-4 as described for the blood injection model.
- Insert the needle (26 Gauge) 3.7 mm ventrally through the burr hole. The needle should remain in position for 10 min before being withdrawn at a rate of 1 mm/min.
- Seal the burr hole with bone wax and suture the skin.
- Inject 0.05 mg/kg of buprenorphine subcutaneously in pre-warmed post-operative fluids.
5. Representative Results
Experimental intrastriatal hemorrhage evokes morphological as well as behavioral changes in rodents. These changes can be evaluated to ensure an adequate execution of the procedure, or to investigate the effects of potential treatments. Generating the bleed in a targeted brain area (e.g. basal ganglia) is most essential for a reproducible approach, and can be verified on gross or histologically stained brain sections (Figure 1-2). Injury to the basal ganglia results in sensorimotor deficits, which can be quantified via various behavioral assessments. Results of the corner turn test showed that, after experimental right-sided ICH, mice turned significantly more often ipsilaterally and away from the impaired contralateral (left) side, than sham operated animals at 24 and 72 hr after surgery (Figure 3 A). Furthermore, the ability to adequately place the impaired (left) forelimb on a surface, following vibrissae stimulation, was evaluated via the forelimb placing test. At 24 and 72 hr after surgery, mice subjected to right-sided ICH showed significantly fewer placements than sham operated animals. Measurement of brain edema is frequently employed to quantify the extent of brain injury after experimental ICH. Intracerebral injections of autologous blood (30 μl) or bacterial collagenase (0.075 U) led to a significant increase of brain water content in the ipsilateral cortex and basal ganglia at 24 (Figure 4 A) and 72 hr (Figure 4 B) after surgery (compared to sham). The outcome of the behavior tests (Figure 3) and the extent of brain edema (Figure 4) showed no difference between the blood and collagenase injection models at given volumes.
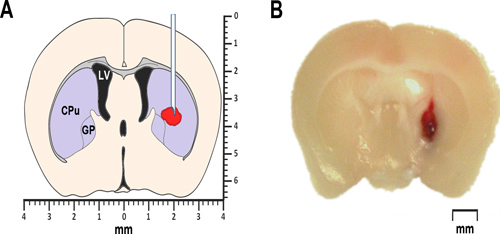
Figure 1. Modeling ICH in mice. (A) The simplified schematic of a coronal brain section 0.2 mm anterior of bregma illustrates the proposed location of autologous blood or collagenase injection. The lateral ventricle is marked LV. CPu stands for caudate-putamen, a part of the striatum, and GP identifies the globus pallidus. Both, the striatum as well as the globus pallidus belong to a group of sub-cortical nuclei, also known as basal ganglia. (B) Representative photomicrograph of a coronal brain section 0.2mm anterior of bregma, obtained at 24 hr after intrastriatal injection of autologous whole blood.
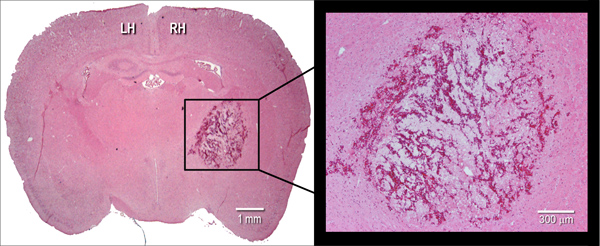
Figure 2. Histological manifestation of the hematoma. Representative hematoxylin and eosin (H&E) stained coronal cryosection (10 μm) of a mouse brain, illustrating hematoma size at 24 hr after intrastriatal injection of bacterial collagenase (0.075 U). LH=left hemisphere, RH=right hemisphere.
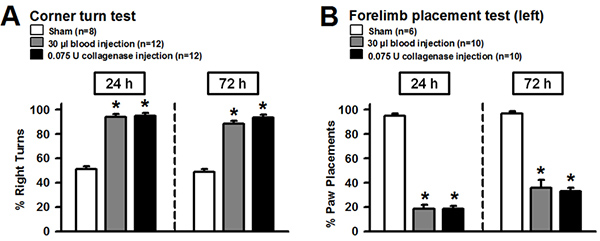
Figure 3. Neurofunctional assessments following experimental ICH in mice. Intrastriatal injection of autologous blood (30 μl) or bacterial collagenase (0.075 U) caused reproducible neurofunctional deficits. (A) Mice after experimental ICH showed significantly more right turns than sham operated animals at 24 and 72 hr after surgery. (B) Forepaw placing capacity of the left limb was impaired after ICH at 24 and 72 hr after surgery. Values were expressed as mean±S.E.M. and analyzed with Kruskal-Wallis One Way Analysis of Variance on Ranks, followed by the Student-Newman-Keuls Method. A P value of <0.05 was considered statistically significant; n=6-12 per group, *P<0.05 compared to sham. Click here to view larger figure.
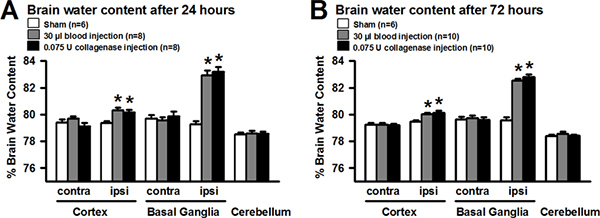
Figure 4. Evaluation of brain water content following experimental ICH in mice. Intracerebral injection of autologous blood (30 μl) or bacterial collagenase (0.075 U) led to significant increase of brain water content in the ipsilateral cortex and basal ganglia at 24 (A) and 72 hr (B) after ICH-induction. Values were expressed as mean±S.E.M. and analyzed with One Way Analysis of Variance, followed by Tukey post hoc test. A P value of <0.05 was considered statistically significant; n=6-10 per group, *P<0.05 compared to sham. Click here to view larger figure.
Dyskusje
Animal models of intracerebral hemorrhage (ICH) contribute greatly to an advanced understanding of the disease's pathophysiology, and are commonly used to develop and evaluate novel therapeutic strategies in a preclinical setting. Intraparenchymal injections of autologous blood or bacterial collagenase are well-established methods to generate ICH in rodents. Both methods were initially developed in the rat; however, due to the rapidly increasing availability of transgenic and knockout strains, mice became indispensable t...
Ujawnienia
No conflicts of interest declared.
Podziękowania
This study was partially supported by NIH grant RO1NS053407 to J.H. Zhang. We would like to thank Mr. Damon Klebe for his valuable contributions.
Materiały
| Name | Company | Catalog Number | Comments |
| Stereotactic Head Frame | Stoelting Co. | 51600 | |
| Nanomite Syringe Pump | Harvard Apparatus | PY2 70-2217 | |
| Hamilton Syringe | Hamilton Company | 1725RN (250 μl) 1701 RN (10 μl) | 26 Gauge needle for 250 μl and 10 μl syringes. |
| Microdrill | Fine Science Tools | 18000-17 | |
| Microdrill burr | Fine Science Tools | 19007-09 | 0.9 mm diameter |
| Collagenase Type VII-S | Sigma-Aldrich | C2399 | |
| Microhematocrit Capillary Tubes | Fisher Scientific | 22-362-574 | unheparinized |
| Bone Wax | Ethicon | W31 | |
| Suture | Ethicon | 1676G | |
| Ketamine | JHP Pharmaceuticals | 42023-115-10 | Ketalar |
| Xylazine | LLOYD Laboratories | 139-236 | AnaSed |
Odniesienia
- Broderick, J. P. Guidelines for the management of spontaneous intracerebral hemorrhage: A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 30, 905-915 (1999).
- Qureshi, A. I., Mendelow, A. D., Hanley, D. F. Intracerebral haemorrhage. Lancet. 373, 1632-1644 (2009).
- MacLellan, C. L., Silasi, G., Auriat, A. M., Colbourne, F. Rodent models of intracerebral hemorrhage. Stroke. 41, 95-98 (2010).
- James, M. L., Warner, D. S., Laskowitz, D. T. Preclinical models of intracerebral hemorrhage: a translational perspective. Neurocrit Care. 9, 139-152 (2008).
- MacLellan, C. L. Intracerebral hemorrhage models in rat: comparing collagenase to blood infusion. J. Cereb. Blood Flow Metab. 28, 516-525 (2008).
- Belayev, L. Experimental intracerebral hemorrhage in the mouse: histological, behavioral, and hemodynamic characterization of a double-injection model. Stroke. 34, 2221-2227 (2003).
- Clark, W., Gunion-Rinker, L., Lessov, N., Hazel, K. Citicoline treatment for experimental intracerebral hemorrhage in mice. Stroke. 29, 2136-2140 (1998).
- Nakamura, T. Intracerebral hemorrhage in mice: model characterization and application for genetically modified mice. J. Cereb. Blood Flow Metab. 24, 487-494 (2004).
- Schallert, T. Behavioral tests for preclinical intervention assessment. NeuroRx. 3, 497-504 (2006).
- Hartman, R., Lekic, T., Rojas, H., Tang, J., Zhang, J. H. Assessing functional outcomes following intracerebral hemorrhage in rats. Brain Res. 1280, 148-157 (2009).
- Hua, Y. Behavioral tests after intracerebral hemorrhage in the rat. Stroke. 33, 2478-2484 (2002).
- Tang, J. Mmp-9 deficiency enhances collagenase-induced intracerebral hemorrhage and brain injury in mutant mice. J. Cereb. Blood Flow Metab. 24, 1133-1145 (2004).
- Ma, Q. Vascular adhesion protein-1 inhibition provides antiinflammatory protection after an intracerebral hemorrhagic stroke in mice. J. Cereb. Blood Flow Metab. , (2010).
- Bullock, R., Mendelow, A. D., Teasdale, G. M., Graham, D. I. Intracranial haemorrhage induced at arterial pressure in the rat. Part 1: Description of technique, ICP changes and neuropathological findings. Neurol Res. 6, 184-188 (1984).
- Yang, G. Y., Betz, A. L., Chenevert, T. L., Brunberg, J. A., Hoff, J. T. Experimental intracerebral hemorrhage: relationship between brain edema, blood flow, and blood-brain barrier permeability in rats. J. Neurosurg. 81, 93-102 (1994).
- Rosenberg, G. A., Mun-Bryce, S., Wesley, M., Kornfeld, M. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 21, 801-807 (1990).
- Alkayed, N. J. Gender-linked brain injury in experimental stroke. Stroke. 29, 159-165 (1998).
- Saha, J. K., Xia, J., Grondin, J. M., Engle, S. K., Jakubowski, J. A. Acute hyperglycemia induced by ketamine/xylazine anesthesia in rats: mechanisms and implications for preclinical models. Exp Biol Med. (Maywood). 230, 777-784 (2005).
- Fujiwara, N. Effect of normobaric oxygen therapy in a rat model of intracerebral hemorrhage. Stroke. 42, 1469-1472 (2011).
- Khatibi, N. H. Isoflurane posttreatment reduces brain injury after an intracerebral hemorrhagic stroke in mice. Anesth Analg. 113, 343-348 (2011).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone