Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Fluorescence Biomembrane Force Probe: Concurrent Quantitation of Receptor-ligand Kinetics and Binding-induced Intracellular Signaling on a Single Cell
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
We describe a technique for concurrently measuring force-regulated single receptor-ligand binding kinetics and real-time imaging of calcium signaling in a single T lymphocyte.
Streszczenie
Membrane receptor-ligand interactions mediate many cellular functions. Binding kinetics and downstream signaling triggered by these molecular interactions are likely affected by the mechanical environment in which binding and signaling take place. A recent study demonstrated that mechanical force can regulate antigen recognition by and triggering of the T-cell receptor (TCR). This was made possible by a new technology we developed and termed fluorescence biomembrane force probe (fBFP), which combines single-molecule force spectroscopy with fluorescence microscopy. Using an ultra-soft human red blood cell as the sensitive force sensor, a high-speed camera and real-time imaging tracking techniques, the fBFP is of ~1 pN (10-12 N), ~3 nm and ~0.5 msec in force, spatial and temporal resolution. With the fBFP, one can precisely measure single receptor-ligand binding kinetics under force regulation and simultaneously image binding-triggered intracellular calcium signaling on a single live cell. This new technology can be used to study other membrane receptor-ligand interaction and signaling in other cells under mechanical regulation.
Wprowadzenie
Cell-to-cell and cell-to-extracellular matrix (ECM) adhesion is mediated by binding between cell surface receptors, ECM proteins, and/or lipids1. Binding allows cells to form functional structures1, as well as recognize, communicate, and react to the environment1-3. Unlike soluble proteins (e.g., cytokines and growth factors) that bind from a three-dimensional (3D) fluid phase onto the cell surface receptors, cell adhesion receptors form bonds with their ligands across a narrow junctional gap to bridge two opposing surfaces that constrain molecular diffusion in a two dimensional (2D) interface4-7. In contrast to 3D kinetics that are commonly measured by traditional binding assays (e.g., surface plasmon resonance or SPR), 2D kinetics have to be quantified with specialized techniques such as atomic force microscopy (AFM)8-10, flow chamber11,12, micropipette13,14, optical tweezers15 and biomembrane force probe (BFP)16-21.
More than merely providing physical linkage for cellular cohesion, adhesion molecules are a major component of the signaling machinery for the cell to communicate with its surroundings. There has been increasing interest in understanding how ligand engagement of adhesion molecules initiates intracellular signaling and how the initial signal is transduced inside the cell. Intuitively, properties of receptor-ligand binding can impact the signals it induces. However, it is difficult to dissect mechanistic relationships between the extracellular interaction and intracellular signaling events using traditional ensemble of biochemical assays because of their many limitations, e.g., a poor temporal resolution and the complete lack of spatial resolution. Existing methods that allow both biophysical (2D receptor-ligand binding kinetics) and biochemical (signaling) observations on live cells include substrates of tunable rigidity22, elastomer pillar arrays23 and flow chamber/microfluidic devices incorporated with fluorescence capability24-26. However, readouts of signaling and receptor-ligand binding have to be obtained separately (most often by different methods), making it difficult to dissect temporal and spatial relations of bond characteristics with signaling events.
Conventional BFP is an ultrasensitive force spectroscopy with high spatiotemporal resolution17. It uses a flexible red blood cell (RBC) as a force sensor, enabling measurement of single-molecule 2D kinetics, mechanical properties and conformational changes14,16,19-21,27-29. A fluorescent imaging based BFP (fBFP) correlates the receptor-ligand binding kinetics with the binding-triggered cell signaling at single-molecule scale. With this setup, in situ cell signaling activities in the context of surface mechanical stimulation was observed in T-cells27. The fBFP is versatile and can be used for studies of cell adhesion and signaling mediated by other molecules in other cells.
Protokół
This protocol follows the guidelines of and has been approved by the human research ethics committee of Georgia Institute of Technology.
1. Human RBCs Isolation, Biotinylation and Osmolarity Adjustment
Note: Step 1.1 should be performed by a trained medical professional such as a nurse, with an Institutional Review Board approved protocol.
- Obtain 8-10 µl (one drop) of blood from finger prick and add to 1 ml of the carbonate/bicarbonate buffer (Table 1 and 2). Gently vortex or pipette the mixture and centrifuge for 1 min at 900 x g. Discard supernatant and wash once more.
- In a small beaker weigh 3.5-4 mg of Biotin-PEG3500-NHS linker (Table 1). Dissolve it in the carbonate/bicarbonate buffer to make the final concentration 3 mg/ml.
- Mix 171 µl of carbonate/bicarbonate buffer, 10 µl of RBC pack and 1049 µl of Biotin-PEG3500-NHS linker solution and incubate at RT for 30 min. Wash the RBC once with carbonate/bicarbonate buffer and then twice with N2-5% buffer (Table 1 and 2).
- Meanwhile, place the linker bottle with loosened cap in a glass vacuum desiccator filled with desiccants in the bottom and vacuum for 5 min, and fill the desiccator with argon. Tighten the cap and take the bottle out. Seal the bottle with plastic paraffin film (Table 1), place it into a container filled with desiccants on the bottom and store in -20 °C.
Note: The steps that involve the use of Biotin-PEG3500-NHS linker, including 1.2-1.4 (except for the incubation and wash in 1.3), need to be performed as fast as possible. - Dilute nystatin into N2-5% buffer to make a final concentration of 40 µg/ml. Mix 5 µl of biotinylated RBC with 71.4 µl of nystatin (Table 1) solution and incubate for 1 hr at 0 °C. Wash twice with N2-5% buffer and store with N2-5% buffer + 0.5% BSA (Table 1) in the refrigerator (4 °C).
2. Glass Bead Silanization
- Cleaning of Bead Surface
- Weigh 50 mg of glass beads powder and re-suspend them in 500 µl of DI water.
- Mix 0.5 ml of 30% H2O2 (Table 1) with 9.5 ml of DI water in a 50 ml beaker, then add 2 ml of concentrated NH4OH (Table 1) and bring this solution to a boiler on a hot plate.
- Add the glass beads to the boiling solution and continue to boil for another 5 min. Gently swirl the solution every min.
- After boiling, transfer ~5 ml of this hot bead suspension into a 15 ml micro-centrifuge tube and top up with RT DI water. Centrifuge at 3,500 x g for 5 min, remove and discard the supernatant.
- Transfer another 5 ml of hot bead suspension and add to the washed beads, top up with more DI water, mix well, and centrifuge again. Repeat this procedure until about 50 ml of DI water is used, which will be a total of 4 to 5 times of washing.
- Transfer the bead suspension into a 1 ml vial. Repeat washing the beads with methanol (Table 1) by centrifugation at 17,000 x g for 5 min for 3 times, and finally re-suspend the beads in 1 ml of 100% methanol.
- Bead Surface Thiolation
- To a 50 ml centrifuge tube add 45.6 ml of methanol, 0.4 ml of acetic acid (Table 1), 1.85 ml of DI water, 1.15 ml of 3-Mercaptopropyltrimethoxysilane (MPTMS) (Table 1) and 1 ml beads suspension prepared in 2.1, then incubate at RT for 3 hr.
- After the reaction, remove all the reactants by washing once with fresh methanol, and re-suspend the beads into 500 µl of methanol. Evenly divide this concentrated glass bead suspension into a set of 20 dry and clean glass vials with screw caps. Evaporate off the methanol by using a jet of dry argon and slowly rotate the vials so as to make a thin layer of dry beads on the sides of each vial.
- Place the vials of beads into a pre-heated drying oven at 120 °C for 5 min and then take out and quickly place the cap(s) loosely on. Place the vials in a glass vacuum desiccator filled with desiccants in the bottom and vacuum the desiccator with a vacuum pump until cooled.
- Purge the vacuumed desiccator with dry argon to bring the desiccator to normal atmospheric pressure. Remove the desiccator lid and quickly re-tighten the cap(s) on the vial. Seal the vials with plastic paraffin film and store them at RT in a dry dark storage box.
- Upon immediate use, take one vial of dry beads and wash once with phosphate buffer (Tables 1 and 2), re-suspend into 50 µl of phosphate buffer and store at 4 °C. This concentrated bead preparation will be referred to as “MPTMS beads” in the following steps.
Note: With proper storage, MPTMS beads could remain functional for up to three months.
3. Bead Functionalization
- Covalently Coating Proteins on Beads
- Take a certain volume (e.g., 2.5 µl) of the protein stock and mix with equal volume of carbonate/bicarbonate buffer to make Solution 1.
Note: The volume depends on the stock concentration and the desired final site density of the protein on the beads surface. - In a small beaker weigh 2-3 mg of MAL-PEG3500-NHS linker (Table 1) and dissolve it with carbonate/bicarbonate buffer to reach a final concentration of 0.231 mg/ml.
- Mix Solution 1 with an equal volume of the linker solution prepared in 3.1.2. Incubate the mixture at RT for 30 min to make Solution 2.
- Meanwhile, place the linker bottle with loosened cap in a glass vacuum desiccator filled with desiccants in the bottom and vacuum for 5 min, and fill the desiccator with argon. Tighten the cap and take the bottle out. Seal the bottle with plastic paraffin film, place it into a container filled with desiccants on the bottom and store in -20 °C.
Note: The steps that involve the use of MAL-PEG3500-NHS linker, including 3.1.2-3.1.4 (except for the incubation in 3.1.3), need to be accomplished as fast as possible. - Mix 5 µl of MPTMS beads with Solution 2 and add phosphate buffer (Table 1) to make a final volume of 250 µl.
- Incubate the beads overnight at RT, wash 3 times with phosphate buffer, and re-suspend into 100 µl of phosphate buffer and store at 4 °C.
- Take a certain volume (e.g., 2.5 µl) of the protein stock and mix with equal volume of carbonate/bicarbonate buffer to make Solution 1.
- Preparing Protein/Streptavidin (SA) Coated Beads
- Follow protocol 3.1.1-3.1.4.
- Mix 5 µl of MPTMS beads with Solution 2 and 5 µl of 4 mg/ml Streptavidin−Maleimide (SA-MAL) (Table 1) solution and then add phosphate buffer to make a final volume of 250 µl.
- Incubate the beads overnight at RT, wash 3 times with phosphate buffer, and finally re-suspend into 100 µl of phosphate buffer and store at 4 °C.
- Coating Streptavidin onto Glass Beads
- Mix 5 µl of MPTMS beads with 5 µl of 4 mg/ml SA solution and add 140 µl of phosphate buffer.
- Incubate the beads overnight at RT, wash 3 times with phosphate buffer, and re-suspend into 50 µl of phosphate buffer and store at 4 °C.
- Coating the SA Coated Beads with a Biotinylated Protein
- Mix 5 µl of SA coated beads with the protein (volume depending on the desired coating density) and add phosphate buffer to make the final volume to be 100 µl.
- Incubate the mixture overnight at 4 °C or for 3 hr at RT, wash 3 times with phosphate buffer, and re-suspend into 50 µl phosphate buffer and store at 4 °C.
4. Cell Preparation
Note: To purify the cells, follow standard cell purification protocols corresponding to the type of cells in use, for example T-cells27 or certain cell lines21,29.
- For fBFP experiments, once the cell suspension is prepared, add Fura2-AM (Table 1) dissolved in DMSO into the cell suspension to reach a final concentration of 2 µM, incubate for 30 min at RT and then wash once. Keep this fluorescently loaded cell suspension in dark until use.
5. Preparation for Micropipettes and a Cell Chamber
- Preparing Micropipettes
- Cut long capillary glass tubes (Table 1) with a glass cutter into short pieces of around 3 inches in length. Mount one piece onto the micropipette puller (Table 1), click the “Pull” button so that the middle of the capillary will be heated by the machine and the capillary will be pulled on the two ends to make two capillaries with sharp tips (raw micropipettes).
Note: By following the product guideline, the desired morphology of the raw pipette has 6-8 mm taper and 0.1-0.5 µm tip. - Mount a raw pipette onto the pipette holder of the micropipette forge (Table 1). Heat to melt the glass sphere on the forge. Insert the tip of the raw pipette inside the glass sphere. Cool down the glass sphere and pull the raw pipette to break it from outside and leave its tip inside the sphere. Repeat this procedure until the desired tip orifice is obtained.
Note: Examples of a micropipette tip inner diameter: 2.0-2.4 µm for a RBC, ~1.5 µm for a bead, ~2-4 µm for a T-cell and -7 µm for a hybridoma cell.
- Cut long capillary glass tubes (Table 1) with a glass cutter into short pieces of around 3 inches in length. Mount one piece onto the micropipette puller (Table 1), click the “Pull” button so that the middle of the capillary will be heated by the machine and the capillary will be pulled on the two ends to make two capillaries with sharp tips (raw micropipettes).
- Building a Cell Chamber
Note: the cell chamber is built on the basis of a home-made chamber holder, which consists of two pieces of metal squares (copper/aluminum) and a handle that links them together (Figure 1D). - Cut a 40 mm x 22 mm x 0.2 mm coverslip using a glass cutter into two 40 mm x 11 mm x 0.2 mm pieces (coverslip 1 and 2). Glue coverslip 1 by grease to the top side of the chamber holder in the way that it bridges the two metal squares, and similarly glue coverslip 2 to the bottom side, which will form a parallel-coverslip cell chamber (Figure 1D).
- Use a pipette to inject 200 µl of experimental buffer in between the two coverslips. Make sure the buffer attaches to both coverslips. Gently rotate and shake the chamber to let the buffer touch both ends of the chamber.
- Carefully inject mineral oil into both sides of the chamber flanking the experimental buffer zone thereby sealing the buffer from the open air. Inject suspensions of probe beads (for example, pMHC-coated beads), RBCs and targets (for example, T-cells) in the upper, middle and lower regions of the buffer zone respectively.
6. BFP experiment
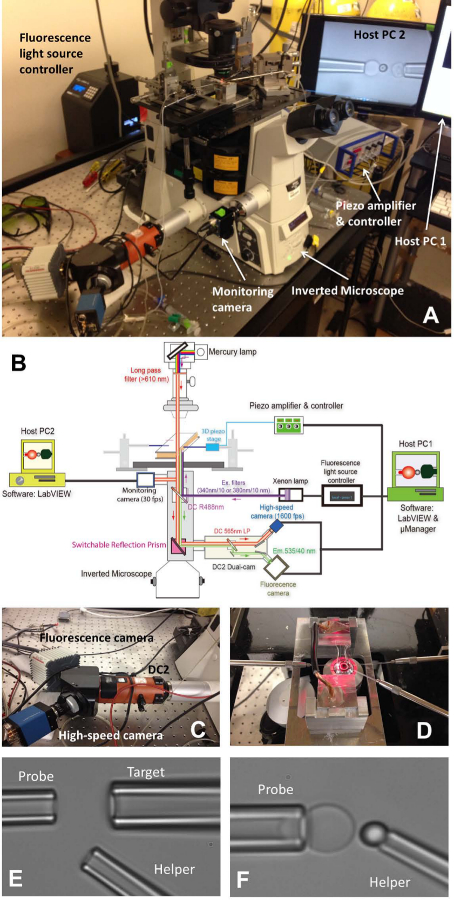
Figure 1: fBFP assembly. (A) An overview picture of the fBFP hardware system. (B) A schematic drawing of the fBFP hardware system. (C) The dual-cam system “DC2” (orange) onto which the high-speed camera (blue) and a fluorescence camera (white) were mounted. (D) The microscope stage that adapts an experiment chamber and three micropipette manipulation systems. (E and F) Micrographs of BFP setting in an experimental chamber. (E) Micropipettes assembly showing the probe pipette (left), target pipette (upper right) and helper pipette (lower right). (F) Probe bead placement. A probe bead was manipulated by a helper pipette and attached to a RBC apex to form a force probe. Please click here to view a larger version of this figure.
- Turn on the microscope (Table 1) and light source. Place the chamber onto the main microscope stage (Figure 1A, D).
- Install all three micropipettes of BFP (Figure 1D. Left: probe, to grab a RBC, right: target, to grab a cell or bead, lower right: helper, to grab a bead).
- Use a micro-injector (Table 1) to backfill a micropipette with experimental buffer. Take off the pipette holder (Table 1) and hold it at a lower place to allow water dripping from the tip. Quickly insert the micropipette into the holder tip and make sure no air bubble gets into the micropipette during the insertion. Tighten the holder screw.
- Mount each pipette holder onto its corresponding micro-manipulator. Push the micropipette towards the chamber so that their tips enter the chamber buffer area. Adjust the position of the micropipette and find them under the microscope field of view.
- Move around the chamber holder stage to find the colonies of three elements (the RBCs, the targets and the probe beads) one-by-one. Adjust the position of the corresponding micropipette by turning the knobs of the manipulators to let the tip of the micropipette approach one cell/bead. Aspirate the cell/bead by adjusting the pressure inside the corresponding micropipette. All three micropipettes will capture their corresponding elements.
- Move around the chamber holder stage to find an open space away from the colonies of the injected elements where the experiment will be performed. Switch the microscope visual mode to visualize the image on the computer program on the computer screen. Move all three elements on pipette tips into the program’s vision field.
- Align the probe bead and RBC, and carefully maneuver the probe bead to the apex of the RBC, briefly impinge the bead onto the RBC and gently retract. Adjust the pressure of the helper micropipette to gently blow the bead away, so that it will be left glued onto the RBC apex (Figure 1F). Move away the helper micropipette and align the target and probe bead (Figure 2A).
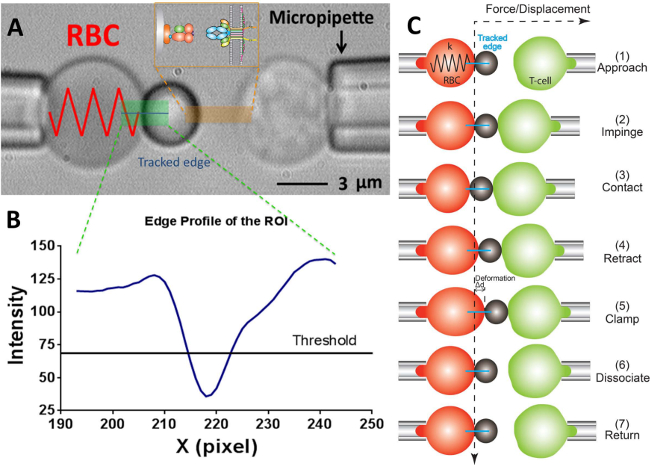
Figure 2: BFP scheme and its test cycle. (A) Video-micrograph depicting a force probe (left) and a target T-cell (right) aspirated by their respective pipettes.The stationary force probe consists of a swollen RBC and an attached ligand-bearing bead. The receptor-bearing T-cell (target) is mounted to a piezotranslator aligned opposite to the probe. The ROI is indicated in green. The edge tracker is indicated in a blue line. The insert depicts the ligand (pMHC, bead side) and receptor (TCR, T-cell side) pair on the two opposing surfaces in the area marked in orange. (B) The intensity profile of the bead edge in (A). The ROI region in the x-direction is plotted as x-axis (in pixel number) and the light intensity (in gray scale value) averaged by binning 30 pixels along the y-direction. (C) The deflection of the RBC and the position of the bead and the target (T-cell) in a test cycle of force clamp assay. The vertical and horizontal dashed lines indicate the zero-force position of the RBC apex and the time course, respectively. The line edge tracker of the RBC deformation is shown in blue in each panel. The same yet less steps are adopted in adhesion frequency assay (which lacks the steps of “clamp” and “dissociate”) and thermal fluctuation assay (which lacks the step of “dissociate”).
- On the program, in the vision field window use the tools in the program to measure the respective radii of the probe micropipette (Rp), the RBC (R0), the circular contact area between the RBC and probe bead (Rc), which allows the estimation of the spring constant of the RBC (k) by the following equation17,30,
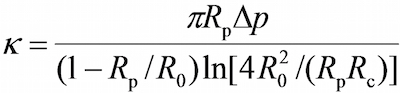
where Δp is the aspiration pressure at probe pipette tip.
Note: It follows from the Hooke's law that the binding force, F, can be quantified by the product of spring constant and displacement of the probe bead (d), i.e., F = d (Figure 2C). - Enter the desired RBC spring constant into the program (Please refer to Protocol section 6.6. The spring constant is typically set at 0.25 or 0.3 pN/nm for the force clamp assay and adhesion frequency assay and 0.1 pN/nm for the thermal fluctuation assay), which will return a required aspiration pressure in the unit of centimeter of water. Adjust the height of the water tank that links to the probe’s pipette until the required aspirating pressure is reached.
- Draw a horizontal line across the RBC apex, which will yield a curve in the adjacent window indicating the brightness (gray scale value) of each pixel along this line. Drag the threshold line to be at around half the depth of the curve (Figure 2A, B).
Note: The minimum point on the brightness curve below the threshold line indicates the position of the bead boundary, thus only one local minimum is allowed. If two or more local minima are at present, it indicates the image is not optimal (likely due to the image being out of focus, or a poor alignment between the probe bead and the RBC). - Select the desired experiment mode: thermal fluctuation assay, adhesion frequency assay or force clamp assay. Set the parameters as desired (e.g., impingement force = 15 pN, loading rate = 1,000 pN/s, contact time = 1 sec, clamping force = 20 pN (for force clamp assay), etc).
- Click “Start”, which allows the program to move the target pipette and drive the target in and out of contact with the probe (see Representative Results section for details). Data collection will be performed in parallel, which records the position of the probe bead in real-time. Stop the program by clicking on the button “Stop experiment”, at which time a window will pop out to allow saving the acquired data.
7. Fluorescence BFP (fBFP) experiment
- To use the fluorescence function of the BFP system, turn on the excitation light source (Table 1) and the fluorescence camera (Table 1), which are controlled by a separate program (Table 1). On the program, select the parameters for the fluorescence imaging, including gain, exposure, excitation channels (in this case, 340 nm and 380 nm light), etc. Follow all preparations in the BFP experiment protocol, including aligning the probe and the target, which will allow for visualization of the target cell live fluorescent image excited by 340 nm or 380 nm excitation light.
- Use the sectioning tool to roughly section the area within which the cell will stay during the entire recording period.
Note: Due to the use of the approach-contact-retraction cycle, the cell will be moving forward and backward repetitively, thus the sectioned area is much larger than the cell itself. - Click on “Record” to allow the 340 nm and 380 nm light to alternately excite the intracellular fluorescence dye (Fura2), and a pair of corresponding fluorescence images will be alternately recorded about once every second. Simultaneously click on “Start” in the program to begin the BFP experiment for analysis molecular interaction and the fluorescence imaging experiment to monitor intracellular calcium signaling. The system will produce a raw data file for the receptor-ligand binding (see Figure 6A below) and a series of fluorescent images in .tiff format for the calcium signals.
8. Data Analysis
- BFP Data Analysis
- Data analysis for adhesion frequency assay
- Sequentially inspect each cycle’s “force vs. time” signal and simply record which cycles contain an adhesion event and which do not, and summarize to yield an average adhesion frequency.
- Collect the rupture force of each adhesion event, which is the peak value of the linearly ramped force before bond rupture. After collecting a sufficient amount of rupture forces in a range of ramp rates, derive the rupture force distribution at each ramp rate from which the force-dependent off-rate of receptor-ligand dissociation is derived using dynamic force spectroscopy analysis18,31.
- Data analysis for thermal fluctuation assay
- Sequentially inspect the clamping phase signal of each cycle, which likely contains multiple bond association and dissociation events. Use the clamping phase thermal fluctuation level (the average standard deviation of a sliding interval of 70 sequential time points of the bead position) in the “force vs. time” signal as a guide to distinguish the bond association and dissociation events, since a bond formation corresponds to a decrease in the thermal fluctuation.
- Designate the interval from the instant of bond dissociation (when the thermal fluctuation resumes to normal level) to the instant of the next bond formation as waiting time, and designate the duration of the bond from its association to dissociation as bond lifetime, which are both collected during the data inspection. Calculate the average waiting time and average bond lifetime, which respectively reflect the reciprocal of on-rate and that of off-rate under zero-force16,30.
- Data analysis for force clamp assay
- Record parameters of all lifetime events including the average force and bond lifetime with the sequence number as well as the starting time and the ending time, which will allow one to draw a cumulative lifetime curve (for example, Figure 6C, yellow curve).
- Collect a sufficient amount of lifetime events under a range of forces. Group them into different force bins, which will produce an average lifetime in each force bin, and altogether yield an “average lifetime vs. force” curve (Figure 4).
- Data analysis for adhesion frequency assay
- Calcium fluorescence Imaging Data Analysis
- Adjust the intensity threshold until the fluorescence images show a clear contour of the cell in both 340 nm and 380 nm channels without background noise (Figure 5A, B). Then review the intracellular Ca2+ signal frame by frame with a pseudo-color indicating the intensity level (Figure 6B), which is derived based on the intensity ratio of 340 nm/380 nm, to generate the “normalized Ca2+ intensity vs. time” curve (Figure 6C). Use the pseudo-color fluorescence images to produce a movie that displays the fluorescence level second by second.
Wyniki
The BFP technique was pioneered by the Evans laboratory in 1995 17. This picoforce tool has been extensively used to measure interactions of proteins immobilized on surfaces, so as to analyze two-dimensional kinetics of adhesion molecules interacting with their ligands16,19,20,30, to measure molecular elasticity21,29, and to determine protein conformational changes21. For an fBFP, an additional set of epi-fluorescence-related devices with the corresponding software system (
Dyskusje
A successful fBFP experiment entails a few critical considerations. First, for the force calculation to be reliable, the micropipette, the RBC, and the probe bead should be aligned as close to coaxial as possible. The projection of the RBC inside the pipette should be about one probe pipette diameter so that the friction between the RBC and the pipette is negligible. For a typical human RBC, the optimal pipette diameter is 2.0-2.4 µm, which yields a best fit of Equation 117,30. Second, to ensure measureme...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
Research related to this paper and the development of the fBFP technology in the Zhu lab were supported by NIH grants AI044902, AI077343, AI038282, HL093723, HL091020, GM096187, and TW008753. We thank Evan Evans for inventing this empowering experimental tool, and members of the Evans lab, Andrew Leung, Koji Kinoshita, Wesley Wong, and Ken Halvorsen, for helping us to build the BFP. We also thank other Zhu lab members, Fang Kong, Chenghao Ge and Kaitao Li, for their helps in the instrumentation development.
Materiały
| Name | Company | Catalog Number | Comments |
| Sodium Phosphate Monobasic Monohydrate (NaH2PO4 • H2O) | Sigma-Aldrich | S9638 | Phosphate buffer preparation |
| Anhy. Sodium Phosphate Dibasic (Na2HPO4) | Sigma-Aldrich | S7907 | Phosphate buffer preparation |
| Sodium Carbonate (Na2CO3) | Sigma-Aldrich | S2127 | Carbonate/bicarbonate buffer preparation |
| Sodium Bicarbonate (NaHCO3) | Sigma-Aldrich | S5761 | Carbonate/bicarbonate buffer preparation |
| Sodium chloride (NaCl) | Sigma-Aldrich | S7653 | N2-5% buffer preparation |
| Potassium chloride (KCl) | Sigma-Aldrich | P9541 | N2-5% buffer preparation |
| Potassium phosphate monobasic (KH2PO4) | Sigma-Aldrich | P5655 | N2-5% buffer preparation |
| Sucrose | Sigma-Aldrich | S0389 | N2-5% buffer preparation |
| MAL-PEG3500-NHS | JenKem | A5002-1 | Bead functionalization |
| Biotin-PEG3500-NHS | JenKem | A5026-1 | RBC biotinylation |
| Nystatin | Sigma-Aldrich | N6261 | RBC osmolarity adjustment |
| Ammonium Hydroxide (NH4OH) | Sigma-Aldrich | A-6899 | Glass bead silanization |
| Methanol | BDH | 67-56-1 | Glass bead silanization |
| 30% Hydrogen Peroxide (H2O2) | J. T. Barker | Jan-86 | Glass bead silanization |
| Acetic Acid (Glacial) | Sigma-Aldrich | ARK2183 | Glass bead silanization |
| 3-Mercaptopropyltrimethoxysilane (MPTMS) | Uct Specialties, llc | 4420-74-0 | Glass bead functionalization |
| Borosilicate Glass beads | Distrilab Particle Technology | 9002 | Glass bead functionalization |
| Streptavidin−Maleimide | Sigma-Aldrich | S9415 | Glass bead functionalization |
| BSA | Sigma-Aldrich | A0336 | Ligand functionalizing |
| Fura2-AM | Life Technologies | F-1201 | Intracellular calcium fluorescence dye loading |
| Dimethyl sulfoxide (DMSO) | Sigma-Aldrich | D2650 | Intracellular calcium fluorescence dye loading |
| Quantibrite PE Beads | BD Biosciences | 340495 | Density quantification |
| Flow Cytometer | BD Biosciences | BD LSR II | Density quantification |
| Capillary Tube 0.7-1.0 mm x 30 inches | Kimble Chase | 46485-1 | Micropipette making |
| Flaming/Brown Micropipette Puller | sutter instrument | P-97 | Micropipette making |
| Pipette microforce | Narishige | MF-900 | Micropipette making |
| Mineral Oil | Fisher Scientific | BP2629-1 | Chamber assembly |
| Microscope Cover Glass | Fisher Scientific | 12-544-G | Chamber assembly |
| Micro-injector | World Precision Instruments | MF34G-5 | Chamber assembly |
| 1 ml syringe | BD | 309602 | chamber assembly |
| Micropipette holder | Narishige | HI-7 | Chamber assembly |
| Home-designed mechanical parts and adaptors fabrications using CNC machining. | Biophysics Instrument | All parts are customized according to the CAD designs. | BFP system |
| Microscope (TiE inverted) | Nikon | MEA53100 | BFP system |
| Objective CFI Plan Fluor 40x (NA 0.75, WD 0.72 mm, Spg) | Nikon | MRH00401 | BFP system |
| Camera, GE680, 640 x 480, GigE, 1/3" CCD, mono | Graftek Imaging | 02-2020C | BFP system |
| Prosilica GC1290 - ICX445, 1/3", C-Mount, 1280 x 960, Mono., CCD, 12 Bit ADC | Graftek Imaging | 02-2185A | BFP system |
| Manual submicron probehead with high resolution remote control | Karl Suss | PH400 | BFP system |
| Anti-vibration table (5’ x 3’) | TMC | 77049089 | BFP system |
| 3D manual translational stage | Newport | 462-XYZ-M | |
| SolidWorks 3D CAD software | SOLIDWORKS Corp. | Version 2012 SP5 | BFP system |
| LabVIEW software | National Instruments | Version 2009 | BFP system, BFP program |
| 3D piezo translational stage | Physik Instrumente | M-105.3P | BFP system |
| Linear piezo accuator | Physik Instrumente | P-753.1CD | BFP system |
| Micromanager software | Version 1.4 | fBFP system, fluorescence imaging program | |
| Dual Cam (DC-2) | Photometrics | 77054724 | fBFP system |
| Dual Cam emission filter (T565LPXR) | Photometrics | 77054725 | fBFP system |
| Fluorescence Camera | Hamamatsu | ORCA-R2 C10600-10B | fBFP system |
| Plastic paraffin film (Parafilm) | Bemis Company, Inc | PM996 | bottle sealing |
| Carbonate/bicarbonate buffer (pH 8.5) | 8.4 g/L sodium carbonate (Na2CO3), 10.6 g/L sodium bicarbonate (NaHCO3) | ||
| Phosphate buffer (pH 6.5-6.8) | 27.6 g/L NaPhosphate monobasic (NaH2PO4 • H2O), 28.4 g/L Anhy. NaPhosphate dibasic (Na2HPO4) | ||
| N2-5% buffer (pH 7.2) | 20.77 g/L potassium chloride (KCl), 2.38 g/L sodium chloride (NaCl), 0.13 g/L potassium phosphate monobasic (KH2PO4), 0.71 g/L anhy. sodium phosphate dibasic (Na2HPO4), 9.70 g/L sucrose |
Odniesienia
- Aplin, A. E., Howe, A., Alahari, S. K., Juliano, R. L. Signal transduction and signal modulation by cell adhesion receptors: the role of integrins, cadherins, immunoglobulin-cell adhesion molecules, and selectins. Pharmacological reviews. 50, 197-263 (1998).
- Davis, M. M., Bjorkman, P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 334, 395-402 (1988).
- Dado, D., Sagi, M., Levenberg, S., Zemel, A. Mechanical control of stem cell differentiation. Regenerative medicine. 7, 101-116 (2012).
- Edwards, L. J., Zarnitsyna, V. I., Hood, J. D., Evavold, B. D., Zhu, C. Insights into T cell recognition of antigen: significance of two-dimensional kinetic parameters. Frontiers in immunology. 3, 86 (2012).
- Zhu, C., Jiang, N., Huang, J., Zarnitsyna, V. I., Evavold, B. D. Insights from in situ analysis of TCR-pMHC recognition: response of an interaction network. Immunological reviews. 251, 49-64 (2013).
- Huang, J., Meyer, C., Zhu, C. T. T cell antigen recognition at the cell membrane. Molecular immunology. 52, 155-164 (2012).
- Zarnitsyna, V., Zhu, C. T. T cell triggering: insights from 2D kinetics analysis of molecular interactions. Physical biology. 9, 045005 (2012).
- Binnig, G., Quate, C. F., Gerber, C. Atomic Force Microscope. Physical Review Letters. 56, 930-933 (1986).
- Marshall, B. T., et al. Direct observation of catch bonds involving cell-adhesion molecules. Nature. 423, 190-193 (2003).
- Kong, F., Garcia, A. J., Mould, A. P., Humphries, M. J., Zhu, C. Demonstration of catch bonds between an integrin and its ligand. The Journal of cell biology. 185, 1275-1284 (2009).
- Yago, T., et al. Catch bonds govern adhesion through L-selectin at threshold shear. The Journal of cell biology. 166, 913-923 (2004).
- Yago, T., et al. Platelet glycoprotein Ibalpha forms catch bonds with human WT vWF but not with type 2B von Willebrand disease vWF. The Journal of clinical investigation. 118, 3195-3207 (2008).
- Chesla, S. E., Selvaraj, P., Zhu, C. Measuring two-dimensional receptor-ligand binding kinetics by micropipette. Biophysical journal. 75, 1553-1572 (1998).
- Huang, J., et al. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature. 464, 932-936 (2010).
- Heinrich, V., Wong, W. P., Halvorsen, K., Evans, E. Imaging biomolecular interactions by fast three-dimensional tracking of laser-confined carrier particles. Langmuir : the ACS journal of surfaces and colloids. 24, 1194-1203 (2008).
- Chen, W., Evans, E. A., McEver, R. P., Zhu, C. Monitoring receptor-ligand interactions between surfaces by thermal fluctuations. Biophysical journal. 94, 694-701 (2008).
- Evans, E., Ritchie, K., Merkel, R. Sensitive force technique to probe molecular adhesion and structural linkages at biological interfaces. Biophysical. 68, 2580-2587 (1995).
- Evans, E., Leung, A., Heinrich, V., Zhu, C. Mechanical switching and coupling between two dissociation pathways in a P-selectin adhesion bond. Proceedings of the National Academy of Sciences of the United States of America. 101, 11281-11286 (2004).
- Ju, L., Dong, J. -. f., Cruz, M. A., Zhu, C. The N-terminal Flanking Region of the A1 Domain Regulates the Force-dependent Binding of von Willebrand Factor to Platelet Glycoprotein Ib. Journal of Biological Chemistry. 288, (2013).
- Chen, W., Lou, J., Zhu, C. Forcing switch from short- to intermediate- and long-lived states of the alphaA domain generates LFA-1/ICAM-1 catch bonds. The Journal of biological chemistry. 285, 35967-35978 (2010).
- Chen, W., Lou, J., Evans, E. A., Zhu, C. Observing force-regulated conformational changes and ligand dissociation from a single integrin on cells. The Journal of cell biology. 199, 497-512 (2012).
- Judokusumo, E., Tabdanov, E., Kumari, S., Dustin, M. L., Kam, L. C. Mechanosensing in T lymphocyte activation. Biophysical journal. 102, L5-L7 (2012).
- Bashour, K. T., et al. CD28 and CD3 have complementary roles in T-cell traction forces. Proceedings of the National Academy of Sciences of the United States of America. 111, 2241-2246 (2014).
- Nesbitt, W. S., et al. Distinct glycoprotein Ib/V/IX and integrin alpha IIbbeta 3-dependent calcium signals cooperatively regulate platelet adhesion under flow. The Journal of biological chemistry. 277, 2965-2972 (2002).
- Mazzucato, M., Pradella, P., Cozzi, M. R., De Marco, L., Ruggeri, Z. M. Sequential cytoplasmic calcium signals in a 2-stage platelet activation process induced by the glycoprotein Ibalpha mechanoreceptor. Blood. 100, 2793-2800 (2002).
- Lefort, C. T., Ley, K. Neutrophil arrest by LFA-1 activation. Frontiers in immunology. 3, 157 (2012).
- Liu, B., Chen, W., Evavold, B. D., Zhu, C. Accumulation of dynamic catch bonds between TCR and agonist peptide-MHC triggers T cell signaling. Cell. 157, 357-368 (2014).
- Lou, J., et al. Flow-enhanced adhesion regulated by a selectin interdomain hinge. The Journal of cell biology. 174, 1107-1117 (2006).
- Fiore, V. F., Ju, L., Chen, Y., Zhu, C., Barker, T. H. Dynamic catch of a Thy-1-alpha5beta1+syndecan-4 trimolecular complex. Nature communications. 5, 4886 (2014).
- Chen, W., Zarnitsyna, V. I., Sarangapani, K. K., Huang, J., Zhu, C. Measuring Receptor-Ligand Binding Kinetics on Cell Surfaces: From Adhesion Frequency to Thermal Fluctuation Methods. Cellular and molecular bioengineering. 1, 276-288 (2008).
- Marshall, B. T., Sarangapani, K. K., Lou, J., McEver, R. P., Zhu, C. Force history dependence of receptor-ligand dissociation. Biophysical. 88, 1458-1466 (2005).
- Xiang, X., et al. Structural basis and kinetics of force-induced conformational changes of an alphaA domain-containing integrin. PloS one. 6, e27946 (2011).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone