Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Measuring Synaptic Vesicle Endocytosis in Cultured Hippocampal Neurons
W tym Artykule
Podsumowanie
Synaptic vesicle endocytosis is detected by light microscopy of pHluorin fused with synaptic vesicle protein and by electron microscopy of vesicle uptake.
Streszczenie
During endocytosis, fused synaptic vesicles are retrieved at nerve terminals, allowing for vesicle recycling and thus the maintenance of synaptic transmission during repetitive nerve firing. Impaired endocytosis in pathological conditions leads to decreases in synaptic strength and brain functions. Here, we describe methods used to measure synaptic vesicle endocytosis at the mammalian hippocampal synapse in neuronal culture. We monitored synaptic vesicle protein endocytosis by fusing a synaptic vesicular membrane protein, including synaptophysin and VAMP2/synaptobrevin, at the vesicular lumenal side, with pHluorin, a pH-sensitive green fluorescent protein that increases its fluorescence intensity as the pH increases. During exocytosis, vesicular lumen pH increases, whereas during endocytosis vesicular lumen pH is re-acidified. Thus, an increase of pHluorin fluorescence intensity indicates fusion, whereas a decrease indicates endocytosis of the labelled synaptic vesicle protein. In addition to using the pHluorin imaging method to record endocytosis, we monitored vesicular membrane endocytosis by electron microscopy (EM) measurements of Horseradish peroxidase (HRP) uptake by vesicles. Finally, we monitored the formation of nerve terminal membrane pits at various times after high potassium-induced depolarization. The time course of HRP uptake and membrane pit formation indicates the time course of endocytosis.
Wprowadzenie
Neurotransmitters are stored in synaptic vesicles and released by exocytosis. The synaptic vesicle membrane and protein are then internalized by endocytosis, and reused in the next round of exocytosis. Endocytosis of synaptic vesicles is important for maintaining synaptic vesicle pools and removes protruding vesicles from the plasma membrane. The pH-sensitive green fluorescent protein pHluorin, which is quenched in acidic circumstances and dequenched in neutral pH, has been used to measure endocytosis time courses in live cells1,2,3. The pHluorin protein is typically attached to the lumenal side of synaptic vesicle proteins, such as synaptophysin or VAMP2/synaptobrevin. At rest, pHluorin is quenched in the 5.5 pH lumen of synaptic vesicles. Vesicle fusion to the plasma membrane exposes the vesicular lumen to the extracellular solution where the pH is ~7.3, resulting in an increase in pHluorin fluorescence. After exocytosis, the increased fluorescence decays, due to endocytosis of synaptic vesicle proteins followed by vesicle re-acidification within those recovered vesicles. Although the decay reflects both endocytosis and vesicular re-acidification, it mostly reflects endocytosis, because re-acidification is faster than endocytosis in most conditions1,4. The time constant of re-acidification is 3-4 s or less5,6, which is generally faster than the 10 s or more required for vesicle endocytosis4,5. If experiments are needed to distinguish endocytosis from re-acidification, acid quenching experiments using the 4-Morpholineethanesulfonic acid (MES) solution (25 mM) with a pH of 5.5 can be used to determine whether synaptic vesicle proteins are retrieved from the plasma membrane via endocytosis1,3,4. Thus, the pHluorin fluorescence intensity increase reflects a balance of exo- and endocytosis, and the decrease after nerve stimulation specifically reflects endocytosis.
pHluorin imaging may be used not only to measure the time course of endocytosis, but also the size of synaptic vesicle pools7,8, and the probability of evoked release and spontaneous release9. Many factors and proteins involved in regulating endocytosis, such as calcium, soluble NSF-attachment protein receptor (SNARE) proteins, brain-derived neurotrophic factor(BDNF), and calcineurin have been identified using pHluorin imaging1,2,10,11,12,13,14,15,16. Moreover, release of neurotransmitter could be detected in not only primary neurons but in neuroblastoma cells with TIRFM17. Recently, pHluorin variants, dsRed, mOrange and pHTomato were developed for monitoring simultaneous recordings of multiple factors in a single synapse18,19. For example, pHTomato has been fused with synaptophysin and used with a genetically encoded calcium indicator (GCaMP5K) to monitor presynaptic vesicle fusion and Ca2+ influx in the postsynaptic compartment20. Therefore, pHluorin attached to synaptic proteins provides a useful method to analyze the relationship between endocytosis and exocytosis.
EM is another method commonly used to study endocytosis, due to the high spatial resolution that shows ultrastructural changes during endocytosis. Two general areas are the ability to visualize pathological changes within neuronal cells21 and track vesicle proteins22. In particular, the observation of synaptic vesicle uptake, membrane curvature coated by clathrin in the periactive zone, and endosomal structures are possible with EM3,23,24,25,26,27,28. While EM involves potential artifacts, such as fixative-induced malformations, that may affect endocytosis, and data analysis is labor intensive, the resolution provides an attractive opportunity to visualize cellular structure. Potential fixative problems and the limitation in EM temporal resolution can be overcome by high pressure freezing, providing a fast and non-chemical method of stabilizing the delicate structures present during endocytosis27.
Protokół
NOTE: The following protocol describes the pHluorin imaging methods and EM methods used in cultured hippocampal neurons. pHluorin monitors synaptic vesicle protein uptake in living cells and EM detects uptake of synaptic vesicle and ultrastructural changes.
Animal care and procedure followed NIH guidelines and were approved by the NIH Animal Care and Use Committee.
1. pHluorin Imaging
- Hippocampal neuron culture
- Prepare Hippocampus Buffer (HB) by combining 4 mM NaHCO3 and 5 mM HEPES and adjust to pH 7.3 with 5 M NaOH. Make the culture medium by mixing neurobasal medium, 2% B27, 0.5 mM L-glutamine and 1% penicillin-streptomycin. Additionally, prepare a mixture of HB buffer with 20% Fetal Bovine Serum (HB/20% FBS).
NOTE: This culturing protocol is based on Sankaranarayanan, et al.29 and Wu, et al.24 - Decapitate mouse pups between postnatal day 0 to day 2 into culture medium, and extract the brain into 4 °C HB/20% FBS. Remove the brainstem and thalamus to expose the hippocampi. Dissect out the hippocampi, after exposing it by removal of the brainstem and thalamus, and transfer to fresh 4 °C HB/20% FBS.
NOTE: Typically, yield is around 4 x 105 cells/mL for one pup (two hippocampi). Clean off the adhering membranes using tweezers or scissors, then transfer to fresh 4 °C HB/20% FBS. Unroll the dentate gyrus and isolate the subiculum by cutting with scissors, then transfer to fresh 4 °C HB/20% FBS. - Divide each hippocampus, by cutting from end to end, into about 10 slices and transfer them to a 15 mL polypropylene conical tube.
- After allowing the tissue to settle, wash with 10 mL of HB/20% FBS, and then wash three times with 10 mL of HB.
- Prepare digestion solution of 137 mM NaCl, 5 mM KCl, 7 mM Na2HPO4, and 25 mM HEPES, and adjust to pH 7.2 with 5 M NaOH. Remove the supernatant from the hippocampi. Add 10 mg of trypsin and 1 mg of DNase to 2 mL of digestion solution, and filter through a sterile 0.22 µm membrane directly onto the sample pellet.
- Incubate the hippocampi for 5 min at 37 °C, then wash twice with 10 mL of HB/20% FBS, and then wash once with 10 mL of HB.
- Dissociate the cells with 6 mg of MgSO4∙7H2O and 1 mg of DNase to 2 mL HB and sterile filter onto the hippocampi pellet through a sterile 0.22 µm filter. Gently dissociate the cells by careful triturating, while taking care to avoid introducing air bubbles. Allow tissue particulates to settle for 2 min, and then slowly transfer the supernatant to another tube.
- Add 3 mL HB/20% FBS to the cell suspension, and centrifuge or 10 min at 4 °C and 1,000 rpm. Discard the supernatant and resuspend in culture medium.
- Plate 60,000 cells suspended in 150 µL culture medium on a Poly-D-Lysine coated 25-mm diameter coverslip, without spill off the coverslip. Add 2 mL of pre-warmed culture medium 2 h after plating. Coverslips are maintained in 6-well culturing plates or sterile Petri dishes.
- Maintain cells at 37 °C in a 5% CO2 humidified incubator in culture medium for 14-21 days prior to recording. During the culture growth, change the top half of the medium twice a week.
- Prepare Hippocampus Buffer (HB) by combining 4 mM NaHCO3 and 5 mM HEPES and adjust to pH 7.3 with 5 M NaOH. Make the culture medium by mixing neurobasal medium, 2% B27, 0.5 mM L-glutamine and 1% penicillin-streptomycin. Additionally, prepare a mixture of HB buffer with 20% Fetal Bovine Serum (HB/20% FBS).
- Transfection
- 6-7 days after plating, transfect synaptophysin-pHluorin 2X (SpH) or VAMP2-pHluorin into hippocampal neurons. For SpH, use a Cytomegalovirus (CMV) promoter, inserted into a pcDNA3 vector30. For VAMP2-pHluorin, use a CMV promoter inserted into a pCI vector31.
- Transfect either vector by lipid carrier into target cells, using 1 µg of plasmid. Use culture medium from protocol step 1.1.1, which lacks serum, for the transfection. Change the medium 2 h after transfection to reduce toxicity.
NOTE: In case of low expression of SpH in the boutons, increase the DNA concentration to 2 µg or the incubation time with the lipid carrier, unless the cells are unhealthy. Typically, 4-10 cells (0.006-0.008%) of neurons were transfected.
- Light microscopy
- Prepare normal saline solution composed of 119 mM NaCl, 2 mM CaCl2, 2.5 mM KCl, 25 mM HEPES (pH 7.4), 30 mM glucose, 2 mM MgCl2, 0.01 mM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), and 0.05 mM DL-2-amino-5-phosphonovaleric acid (AP5). Take a coverslip from the culture medium plate and place on an imaging chamber that allows field stimulation, using lubricant and sealant to avoid leaks. Avoid letting the glass dry during the transfer of a coverslip from the plate to the chamber by immediately adding 750 µL normal saline solution.
NOTE: CNQX and AP5 were used to block postsynaptic activity, which has the potential for recurrent activity. Before placing a chamber on an inverted fluorescence microscope, use cleaning tissue to confirm that the chamber does not leak. Slow leaking causes focus changes during the recording by the mixing of normal saline and immersion oil, which results in changes in the refractive index. - Stimulation and recording
- Image on an inverted widefield microscope with a 60X (1.4 numerical aperture) oil immersion lens with a metal halide lamp. Visualize pHluorin with a filter set for an excitation peak of 480 nm, a 490 nm long pass mirror, a 500-550 nm emission filter, and a manual flip shutter. Capture images every 100 ms, with 2 x 2 binning, using an Electron Multiplying Charge Coupled Device (EMCCD) camera.
NOTE: Several features should be considered to achieve the proper conditions to avoid photobleaching, including the image capture interval, and filter and binning, which are dependent on equipment. In the case of confocal imaging, laser power and exposure time should be considered to avoid photobleaching. The setup was determined after recording at least 3 min without stimulus and the recording was performed for at least 10 s without stimulus to check for photobleaching. - Choose an area with a high density of boutons for ease of analysis in each experiment. Identify transfected cells by their green fluorescence signal, with emphasis on round or oval expression patterns in the bouton and continuous expression between boutons.
- Induce endocytosis by field stimulation using a 1 ms pulse, 20 mA action potential (AP) supplied from a pulse stimulator and delivered through a platinum electrode within the stimulus isolation unit. Image fluorescence activity over the course of the stimulation and during cell recovery.
NOTE: In the case of dead cells, expression was stronger than living boutons and showed a patchy pattern between boutons.
- Image on an inverted widefield microscope with a 60X (1.4 numerical aperture) oil immersion lens with a metal halide lamp. Visualize pHluorin with a filter set for an excitation peak of 480 nm, a 490 nm long pass mirror, a 500-550 nm emission filter, and a manual flip shutter. Capture images every 100 ms, with 2 x 2 binning, using an Electron Multiplying Charge Coupled Device (EMCCD) camera.
- Prepare normal saline solution composed of 119 mM NaCl, 2 mM CaCl2, 2.5 mM KCl, 25 mM HEPES (pH 7.4), 30 mM glucose, 2 mM MgCl2, 0.01 mM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), and 0.05 mM DL-2-amino-5-phosphonovaleric acid (AP5). Take a coverslip from the culture medium plate and place on an imaging chamber that allows field stimulation, using lubricant and sealant to avoid leaks. Avoid letting the glass dry during the transfer of a coverslip from the plate to the chamber by immediately adding 750 µL normal saline solution.
- Image analysis
- For analyzing the fluorescence intensity in a single bouton, set up a Region of Interest (ROI) as a 1.5 x 1.5 µm square; the size of a bouton is within about 1.5 µm. Use traces prior to stimulation to check for photobleaching and subtract as background.
- Normalize fluorescence change (ΔF) with the equation:
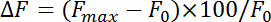
where Fmax and F0 refer to maximal increase after stimulation and baseline fluorescence, respectively. Measure endocytosis rates as the rate of decay during the first 4-10 s after the maximum point of pHluorin fluorescence. Obtain the time constant (τ) of endocytosis by fitting the pHluorin fluorescence decay from the peak increase to the baseline with a mono-exponential function.
2. Electron Microscopy
- Prepare a Poly-D-Lysine coated 6-well plate by applying 1.5 mL of 0.01% sterile-filtered Poly-D-Lysine solution to each well for 1 h at room temperature, then washing three times with sterilized water. Dissect, culture, and maintain hippocampal neurons as in steps 1.1.1, 1.1.2, and 1.1.3, respectively.
- Prepare a high K+ stimulation solution with HRP as 31.5 mM NaCl, 2 mM CaCl2, 90 mM KCl, 25 mM HEPES (pH 7.4), 30 mM glucose, 2 mM MgCl2, 0.01 mM CNQX, 0.05 mM AP5, and 5 mg/mL HRP, then adjust to pH 7.4 with 5 M NaOH.
- Stimulate the hippocampal neuron culture with 1.5 mL high K+ stimulation solution at room temperature (referred to as K+) by addition of 1.5 mL to each well for 90 s. In the resting condition (referred to as R), apply the same concentration of 5 mg/mL HRP for 90 s, but with normal saline solution. For the recovery sample, apply high K+ stimulation solution as with the K+ sample, then rapidly wash and replace with normal saline and incubate for 10 min.
- Fixation and staining
- Prepare 0.1 M Na cacodylate buffer using 21.4 g/L Na cacodylate at pH 7.4. Fix cells with 4% glutaraldehyde in 0.1 M Na cacodylate buffer for at least 1 h at room temperature. Wash three times with 0.1 M Na cacodylate buffer for 7 min each.
- Prepare Diaminobenzidine (DAB) solution, comprised of 0.5 mg/mL of DAB with 0.3% H2O2 in ddH2O, and filter with a 0.22 µm filter. Apply 1.5 mL DAB solution for 30 min at 37 °C. Wash three times with 0.1 M Na cacodylate buffer for 7 min each.
CAUTION: DAB is a toxic and suspected carcinogen. Please use gloves and lab coats.
NOTE: Labeling with DAB occurs due to its oxidation by H2O2, as catalyzed by HRP. Small increases in the components result in an increased signal in the sample. Increasing the concentration of HRP speeds up the effect of the catalyst. Sufficiently large concentrations of H2O2 allow for debilitating side reactions with HRP, inhibiting the effect of labeling32. In this work, the concentrations of this labeling system were chosen based on currently available research33,34. - Incubate the neurons with 1.5 mL of 1% OsO4 in 0.1 M Na cacodylate buffer for 1 h at 4 °C as post fixation. Wash three times with 1.5 mL of 0.1 M Na cacodylate buffer for 7 min each.
CAUTION: Due to the toxicity and reactivity of OsO4, keeping the sample on ice in a chemical hood is preferable in many cases to using a fridge for the incubation. - Prepare 0.1 M sodium acetate buffer with 13.61 g/L sodium acetate and 11.43 mL/L glacial acetic acid at pH 5.0. Wash three times with 1.5 mL 0.1 M acetate buffer at pH 5.0 for 7 min each and incubate with 1.5 mL 1% uranyl acetate in 0.1 M acetate buffer at pH 5.0 for 1 h at 4 °C. Wash three times with 1.5 mL 0.1 M acetate buffer for 7 min each.
- Epoxy embedding
- Dehydrate the neuron culture with single 1.5 mL washes of 50%, 70%, and 90% ethanol, for 7 min in each and then 3 washes of 1.5 mL 100% ethanol for 7 min each in a fume hood.
- Mix 485 mL/L bisphenol-A-(epichlorhydrin) epoxy resin, 160 mL/L dodecenyl succinic anhydride (DDSA), 340 mL/L Methyl-5-Norbornene-2,3-Dicarboxylic Anhydride (NMA), and 15 mL/L 2,4,6-tris(dimethylaminomethyl)phenol (DMP-30) to create the epoxy resin. Mix thoroughly, then store under vacuum to remove air bubbles.
NOTE: It is critical to remove air bubbles in the resin, especially those smaller than visible to the naked eye, because they can cause cavities in the resin during sectioning. - Infiltrate the sample by replacing the ethanol with 50% epoxy resin in ethanol for 30 min at room temperature on a shaker, then 70% epoxy resin in ethanol for 30 min at room temperature on a shaker.
- Switch the epoxy resin solution with 100% epoxy resin and incubate for 10 min at 50 °C. Perform two exchanges of fresh 100% epoxy resin with incubations for 1 h at 50 °C. Add fresh 100% epoxy resin and allow to harden at 50 °C overnight and then at 60 °C for over 36 h to harden.
- Remove each sample from the muti-well plate with a jeweler's handsaw. Select regions of interest, dense concentrations of cells, using an inverted light microscope, and then cut 70 to 80 nm blocks for sectioning by microtome. Mount the cut region in the microtome chuck and load the microtome. Position the chuck in the microtome and mount a diamond knife with the edge parallel to the surface of the block. Collect sections of 70 to 80 nm thickness directly onto individual grids.
- Dissolve uranyl acetate into water for a 1% solution by weight, and separately dissolve lead citrate into water for a 3% solution by weight. Counterstain the sections by submersion with 1% aqueous uranyl acetate for 15 min and then 3% aqueous lead citrate for 5 min to improve the contrast of the samples.
- EM imaging
- Examine the sections with a transmission electron microscope and record images with a CCD digital camera at a primary magnification of 10,000-20,000X3.
- Statistics
- Perform a t-test to identify significant differences by comparing the mean and standard error of measurement (s.e.m.) between the control and experimental samples.
Wyniki
Using the lipid carrier method, SpH was expressed in hippocampal neurons, allowing for the identification of boutons (Figure 1a). Electrical stimulation of the cells induced exocytosis, and a corresponding increase in fluorescence intensity. The increase in fluorescence (ΔF) was stopped by ending the stimulus (Figure 1b). The increased fluorescence was followed by a slow decrease, due to endocytosis. In the case of VAMP2-pHl...
Dyskusje
Here we demonstrate two methods for monitoring synaptic vesicle endocytosis. In the first method, we monitored pHluorin fused with a synaptic vesicle protein in transfected neurons and subsequently electrically stimulated. Secondly, we used EM imaging of HRP uptake as induced by KCl. We used different stimuli for two reasons. First, high potassium application induces depolarization of all neurons in the culture. This facilitates EM examination, given that our EM methodology could not distinguish between non-stimulated an...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
We thank Dr. Yong-Ling Zhu for providing synaptophysin-pHluorin2x construct, and Dr. James E. Rothman for providing VAMP2-phluorin. We thank Dr. Susan Cheng and Virginia Crocker of NINDS Electron Microscopy Facility for their technical support and help. This work was supported by the National Institute of Neurological Disorders and Stroke Intramural Research Program in USA and a grant from the KRIBB Research Initiative Program (Korean Biomedical Scientist Fellowship Program), Korea Research Institute of Bioscience and Biotechnology, Republic of Korea.
Materiały
| Name | Company | Catalog Number | Comments |
| Lipofectamine LTX with Plus | Thermo Fisher | 15338-100 | Transfection of plasmid DNA including synaptophysin or VAMP2-pHluorin |
| neurobasal medium | Thermo Fisher | 21103-049 | Growth medium for neuron, Warm up to 37°C before use |
| B27 | Thermo Fisher | 17504-044 | Gradient for neuronal differentiation |
| Glutamax | Thermo Fisher | 35050-061 | Gradient for neuronal culture |
| Poly-D-Lysine coated coverslip | Neuvitro | GG-25-pdl | Substrate for neuronal growth and imaging of pHluorin |
| Trypsin XI from bovine pancrease | Sigma | T1005 | Neuronal culture-digest hippocampal tissues |
| Deoxyribonuclease I from bovine pancreas | Sigma | D5025 | Neuronal culture-inhibits viscous cell suspension |
| pulse stimulator | A-M systems | model 2100 | Apply electrical stimulation |
| Slotted bath with field stimulation | Warner Instruments | RC-21BRFS | Apply electrical stimulation |
| stimulus isolation unit | Warner Instruments | SIU102 | Apply electrical stimulation |
| lubricant | Dow corning | 111 | pHluorin imaging-seal with coverslip and imaging chamber, avoid leak from chamber |
| AP5 | Tocris | 3693 | Gradient for normal saline, selective NMDA receptor antagonist, inhibit postsynaptic activity which have potential for recurrent activity |
| CNQX | Tocris | 190 | Gradient for normal saline, competitive AMPA/kainate receptor antagonist, inhibit postsynaptic activity which have potential for recurrent activity |
| Illuminator | Nikon | C-HGFI | Metal halide light source for pHluorin |
| EMCCD camera | Andor | iXon3 | pHluorin imaging, detect pHluorin fluorescence intensity |
| Inverted microscopy | Nikon | Ti-E | Imaging for synaptophysin or VAMP2 pHluorin transfected cells |
| NIS-Elements AR | Nikon | NIS-Elements Advanced Research | Software for imaging acquisition and analysis |
| Igor Pro | WaveMetrics | Igor pro | Software for imaging analysis and data presentation |
| imaging chamber | Warner Instruments | RC21B | pHluorin imaging, apply field stimulation on living cells |
| poly-l-lysine | Sigma | P4832 | Electron microscopy, substrate for neuronal growth, apply on multiwell plate for 1 h at room temperature then wash with sterilized water 3 times |
| Horseradish peroxidase(HRP) | Sigma | P6782 | Electron microscopy, labeling of endocytosed synaptic vesicles by catalyzing DAB in presence hydrogen peroxide, final concentration is 5 mg/mL in normal saline, make fresh before use |
| Na cacodylate | Electron Microscopy Sciences | 12300 | Electron microscopy, buffer for fixatives and washing, final concentration is 0.1 N |
| 3,3′-Diaminobenzidine(DAB) | Sigma | D8001 | Electron microscopy, labeling of endocytosed synaptic vesicles, substrate for HRP, final concentration is 0.5 mg/mL in DDW and filtered, make fresh before use |
| Hydrogen peroxide solution | Sigma | H1009 | Electron microscopy, labeling of endocytosed synaptic vesicles by inducing HRP-DAB reaction, final concentration is 0.3% in DDW, make fresh before use |
| glutaraldehyde | Electron Microscopy Sciences | 16365 | Electron microscopy, fixatives, final concentration is 4% in Na-cacodylate buffer, make fresh before use, shake well before to use |
| TEM | JEOL | 200CX | Electron microscopy, imaging of endocytosed vesicles and ultrastructural changes |
| CCD digital camera | AMT | XR-100 | Electron microscopy, capturing images |
| Lead citrate | Leica microsystems | 16707235 | Electron microscopy, grid staining |
Odniesienia
- Sankaranarayanan, S., Ryan, T. A. Real-time measurements of vesicle-SNARE recycling in synapses of the central nervous system. Nature cell biol. 2 (4), 197-204 (2000).
- Sun, T., Wu, X. S., et al. The role of calcium/calmodulin-activated calcineurin in rapid and slow endocytosis at central synapses. J Neurosci. 30 (35), 11838-11847 (2010).
- Wu, X. -. S. S., Lee, S. H., et al. Actin Is Crucial for All Kinetically Distinguishable Forms of Endocytosis at Synapses. Neuron. 92 (5), 1020-1035 (2016).
- Granseth, B., Odermatt, B., Royle, S. J., Lagnado, L. Clathrin-mediated endocytosis is the dominant mechanism of vesicle retrieval at hippocampal synapses. Neuron. 51 (6), 773-786 (2006).
- Atluri, P. P., Ryan, T. A. The kinetics of synaptic vesicle reacidification at hippocampal nerve terminals. J Neurosci. 26 (8), 2313-2320 (2006).
- Royle, S. J., Granseth, B., Odermatt, B., Derevier, A., Lagnado, L. Imaging phluorin-based probes at hippocampal synapses. Methods Mol Biol. 457, 293-303 (2008).
- Moulder, K. L., Mennerick, S. Reluctant vesicles contribute to the total readily releasable pool in glutamatergic hippocampal neurons. J Neurosci. 25 (15), 3842-3850 (2005).
- Li, Z., Burrone, J., Tyler, W. J., Hartman, K. N., Albeanu, D. F., Murthy, V. N. Synaptic vesicle recycling studied in transgenic mice expressing synaptopHluorin. Proc Natl Acad Sci U S A. 102 (17), 6131-6136 (2005).
- Morris, R. G. Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. Eur J Neurosci. 23 (11), 2829-2846 (2006).
- Sankaranarayanan, S., Ryan, T. A. Calcium accelerates endocytosis of vSNAREs at hippocampal synapses. Nat Neurosci. 4 (2), 129-136 (2001).
- Balaji, J., Armbruster, M., Ryan, T. A. Calcium control of endocytic capacity at a CNS synapse. J Neurosci. 28 (26), 6742-6749 (2008).
- Ferguson, S. M., Brasnjo, G., et al. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science. 316 (5824), 570-574 (2007).
- Baydyuk, M., Wu, X. S., He, L., Wu, L. G. Brain-derived neurotrophic factor inhibits calcium channel activation, exocytosis, and endocytosis at a central nerve terminal. J Neurosci. 35 (11), 4676-4682 (2015).
- Wu, X. S., Zhang, Z., Zhao, W. D., Wang, D., Luo, F., Wu, L. G. Calcineurin is universally involved in vesicle endocytosis at neuronal and nonneuronal secretory cells. Cell Rep. 7 (4), 982-988 (2014).
- Zhang, Z., Wang, D., et al. The SNARE proteins SNAP25 and synaptobrevin are involved in endocytosis at hippocampal synapses. J Neurosci. 33 (21), 9169-9175 (2013).
- Wu, L. -. G. G., Hamid, E., Shin, W., Chiang, H. -. C. C. Exocytosis and endocytosis: modes, functions, and coupling mechanisms. Annu Rev Physiol. 76 (1), 301-331 (2014).
- Daniele, F., Di Cairano, E. S., Moretti, S., Piccoli, G., Perego, C. TIRFM and pH-sensitive GFP-probes to evaluate neurotransmitter vesicle dynamics in SH-SY5Y neuroblastoma cells: cell imaging and data analysis. J Vis Exp. (95), e52267 (2015).
- Shaner, N. C., Lin, M. Z., et al. Improving the photostability of bright monomeric orange and red fluorescent proteins. Nat Methods. 5 (6), 545-551 (2008).
- Li, Y., Tsien, R. W. pHTomato, a red, genetically encoded indicator that enables multiplex interrogation of synaptic activity. Nat Neurosci. 15 (7), 1047-1053 (2012).
- Leitz, J., Kavalali, E. T. Fast retrieval and autonomous regulation of single spontaneously recycling synaptic vesicles. Elife. 3, e03658 (2014).
- Bisht, K., El Hajj, H., Savage, J. C., Sánchez, M. G., Tremblay, M. -. &. #. 2. 0. 0. ;. Correlative Light and Electron Microscopy to Study Microglial Interactions with β-Amyloid Plaques. J Vis Exp. (112), e54060 (2016).
- Schikorski, T. Monitoring Synaptic Vesicle Protein Sorting with Enhanced Horseradish Peroxidase in the Electron Microscope. High-Resolution Imaging of Cellular Proteins: Methods and Protocols. , 327-341 (2016).
- Kononenko, N. L., Puchkov, D., et al. Clathrin/AP-2 mediate synaptic vesicle reformation from endosome-like vacuoles but are not essential for membrane retrieval at central synapses. Neuron. 82 (5), 981-988 (2014).
- Wu, Y., O'Toole, E. T., et al. A dynamin 1-, dynamin 3- and clathrin-independent pathway of synaptic vesicle recycling mediated by bulk endocytosis. eLife. 2014 (3), e01621 (2014).
- Heuser, J. E., Reese, T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 57 (2), 315-344 (1973).
- Ceccarelli, B., Hurlbut, W. P., Mauro, A. Turnover of transmitter and synaptic vesicles at the frog neuromuscular junction. J Cell Biol. 57 (2), 499-524 (1973).
- Watanabe, S., Rost, B. R., et al. Ultrafast endocytosis at mouse hippocampal synapses. Nature. 504 (7479), 242-247 (2013).
- Watanabe, S., Trimbuch, T., et al. Clathrin regenerates synaptic vesicles from endosomes. Nature. 515 (7526), 228-233 (2014).
- Sankaranarayanan, S., Atluri, P. P., Ryan, T. A. Actin has a molecular scaffolding, not propulsive, role in presynaptic function. Nat Neurosci. 6 (2), 127-135 (2003).
- Zhu, Y., Xu, J., Heinemann, S. F. Two pathways of synaptic vesicle retrieval revealed by single-vesicle imaging. Neuron. 61 (3), 397-411 (2009).
- Miesenbock, G., De Angelis, D. A., Rothman, J. E. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 394 (6689), 192-195 (1998).
- Arnao, M. B. B., Acosta, M., del Rio, J. A. A., García-Cánovas, F. Inactivation of peroxidase by hydrogen peroxide and its protection by a reductant agent. Biochim Biophys Acta. 1038 (1), 85-89 (1990).
- Deák, F., Schoch, S., et al. Synaptobrevin is essential for fast synaptic-vesicle endocytosis. Nat Cell Biol. 6 (11), 1102-1108 (2004).
- Clayton, E. L., Evans, G. J. O., Cousin, M. A. Bulk synaptic vesicle endocytosis is rapidly triggered during strong stimulation. J Neurosci. 28 (26), 6627-6632 (2008).
- Kavalali, E. T., Jorgensen, E. M. Visualizing presynaptic function. Nat Neurosci. 17 (1), 10-16 (2014).
- Wienisch, M., Klingauf, J. Vesicular proteins exocytosed and subsequently retrieved by compensatory endocytosis are nonidentical. Nat Neurosci. 9 (8), 1019-1027 (2006).
- Fernández-Alfonso, T., Kwan, R., Ryan, T. A. Synaptic vesicles interchange their membrane proteins with a large surface reservoir during recycling. Neuron. 51 (2), 179-186 (2006).
- Gimber, N., Tadeus, G., Maritzen, T., Schmoranzer, J., Haucke, V. Diffusional spread and confinement of newly exocytosed synaptic vesicle proteins. Nat Commun. 6, 8392 (2015).
- Nicholson-Fish, J. C., Smillie, K. J., Cousin, M. A. Monitoring activity-dependent bulk endocytosis with the genetically-encoded reporter VAMP4-pHluorin. J Neurosci Methods. 266, 1-10 (2016).
- Burrone, J., Li, Z., Murthy, V. N. Studying vesicle cycling in presynaptic terminals using the genetically encoded probe synaptopHluorin. Nat Protoc. 1 (6), 2970-2978 (2006).
- Wisse, E., Braet, F., et al. Fixation methods for electron microscopy of human and other liver. World journal of gastroenterology. 16 (23), 2851-2866 (2010).
- Magidson, V., Khodjakov, A. Circumventing Photodamage in Live-Cell Microscopy. Methods in Cell Biology. 114, 545-560 (2013).
- Søndergaard, C. R., Garrett, A. E., et al. Structural artifacts in protein-ligand X-ray structures: implications for the development of docking scoring functions. J Med Chem. 52 (18), 5673-5684 (2009).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone