Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
A Small-Scale Setup for Algal Toxicity Testing of Nanomaterials and Other Difficult Substances
W tym Artykule
Podsumowanie
We demonstrate algal toxicity testing for difficult substances (e.g., colored substances or nanomaterials) using a setup illuminated vertically with an LED.
Streszczenie
Ecotoxicity data is a requirement for pre- and post-market registration of chemicals by European and international regulations (e.g., REACH). The algal toxicity test is frequently used in regulatory risk assessment of chemicals. In order to achieve high reliability and reproducibility the development of standardized guidelines is vital. For algal toxicity testing, the guidelines require stable and uniform conditions of parameters such as pH, temperature, carbon dioxide levels and light intensity. Nanomaterials and other so-called difficult substances can interfere with light causing a large variation in results obtained hampering their regulatory acceptance. To address these challenges, we have developed LEVITATT (LED Vertical Illumination Table for Algal Toxicity Tests). The setup utilizes LED illumination from below allowing for a homogenous light distribution and temperature control while also minimizing intra-sample shading. The setup optimizes the sample volume for biomass quantification and does at the same time ensure a sufficient influx of CO2 to support exponential growth of the algae. Additionally, the material of the test containers can be tailored to minimize adsorption and volatilization. When testing colored substances or particle suspensions, the use of LED lights also allows for increasing the light intensity without additional heat generation. The compact design and minimal equipment requirements increase the possibilities for implementation of the LEVITATT in a wide range of laboratories. While compliant with standardized ISO and OECD guidelines for algal toxicity testing, LEVITATT also showed a lower inter-sample variability for two reference substances (3,5-Dicholorophenol and K2Cr2O7) and three nanomaterials (ZnO, CeO2, and BaSO4) compared to Erlenmeyer flasks and microtiter plates.
Wprowadzenie
The algal toxicity test is one of only three mandatory tests used to generate the ecotoxicity data required for pre- and post-market registration of chemicals by European and international regulations (e.g., REACH1 and TSCA (USA)). For this purpose, standardized algal test guidelines have been developed by international organizations (e.g., ISO and OECD). These testing standards and guidelines prescribe ideal test conditions in terms of pH, temperature, carbon dioxide levels and light intensity. However, maintaining stable test conditions during algal testing is in practice difficult and the results suffer from problems with reproducibility and reliability for a range of chemical substances and nanomaterials (often referred to as “difficult substances”)2. Most of the existing algal toxicity testing setups operate with relatively large volumes (100–250 mL) situated on an orbital shaker inside an incubator. Such a setup limits the number of test concentrations and replicates achievable and high volumes of algal culture and test material. Additionally, these setups rarely have a uniform light field and reliable lighting conditions are furthermore difficult to obtain in large flasks, partly as light intensity decreases exponentially the further the light travels and partly due to the flask geometry. Alternative setups comprise plastic microtiter3 plates containing small sample volumes that do not allow for adequate sampling volumes to measure pH, additional biomass measurements, pigment extraction or other analyses requiring destructive sampling. One particular challenge using existing setups for algal toxicity testing of nanomaterials and substances forming colored suspensions is the interference or blocking of the light available to the algal cells, often referred to as “shading”4,5. Shading may occur within vials by the test material and/or interactions between the test material and the algal cells, or shading can occur between vials, due to their positioning relative to each other and the light source.
The method is based on the small-scale algal toxicity test setup introduced by Arensberg et al.6 that allows for testing in compliance with standards such as OECD 2017, and ISO 86928. The method is further optimized to address the limitations stated above by: 1) utilizing the LED light technology to ensure uniform light conditions with minimal heat generation, 2) providing adequate sample volume for chemical/biological analysis while maintaining constant pH, CO2 levels, and 3) enabling the use of versatile test container material for testing of volatile substances or substances with a high sorption potential.
Protokół
1. Description of the LEVITATT setup
- Use 20 mL scintillation glass vials (Figure 1, insert 1) allowing light penetration. Alternatively, light penetrable plastic vials can be used. Quantify the light intensity using a photometer.
- Use at least a 4 mL test suspension at the beginning of the test to allow for quantification of biomass and for characterization/quantification of nanomaterials during and after incubation (Figure 1, insert 2).
- Fit the 20 mL scintillation vials with a cap (Figure 1, insert 3) where a small hole is drilled (approximately 1 mm in diameter) to allow for CO2 exchange with the atmosphere. This exchange is crucial to ensure stable pH and CO2 levels during testing.
- For volatile substances, use an air-tight Teflon coated cap to allow for CO2 enriching of the headspace using a syringe9 or completely closed flasks with no gas phase in which CO2 is maintained in solution by an enriched sodium bicarbonate (NaHCO3) buffer system10.
- Fasten the vials with clamps mounted on the exterior casing (Figure 1, insert 4).
- Use an LED light source located below the test vials (Figure 1, insert 5) providing a uniform fluorescent illumination of “cool-white” or “daylight” type and a light intensity in the range 60–120 µE∙m-2∙s-1 measured in the photosynthetically effective wavelength range of 400 nm to 700 nm. The setup employs adjustable light intensity in the range 5–160 µE∙m-2∙s-1 by fitting a light dimmer to the source. This allows for testing at higher and lower light intensities.
- Mount the setup on an orbital shaker to agitate samples throughout the duration of the test. This keeps the cells in free suspension and facilitates CO2 mass transfer from air to water (Figure 1, insert 6).
- Place the setup in a temperature-controlled room or a thermostatic cabinet to maintain stable temperatures throughout testing (Figure 1, insert 7).
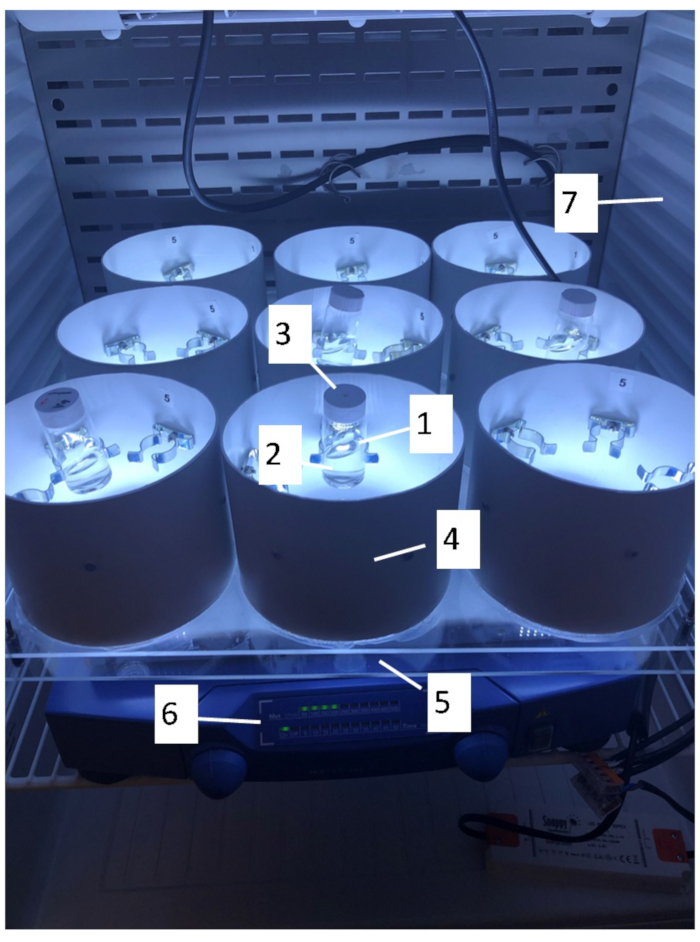
Figure 1: Picture of LED Vertical Illumination Table for Algal Toxicity Tests (LEVITATT). 1) 20 mL glass scintillation vials for incubation, 2) 4 mL sample for analysis, 3) lid with drilled hole for CO2 exchange, 4) casing for defined light conditions, 5) LED light source located in the center of the casing, 6) orbital shaker for agitation during the experiment, and 7) a thermostatic cabinet. Please click here to view a larger version of this figure.
2. Preparation of algal growth medium
- The ISO 8692 algal growth medium consists of four different stock solutions. Weigh out the appropriate amount of salts and dilute in ultrapure water according to Table 1.
| Stock solutions | Nutrient | Concentration in stock solution | Concentration in test solution |
| 1: Macronutrients | NH4Cl | 1.5 g/L | 15 mg/L (N: 3.9 mg/L) |
| MgCl2∙6H2O | 1.2 g/L | 12 mg/L (Mg: 2.9 mg/L) | |
| CaCl2∙2H2O | 1.8 g/L | 18 mg/L (Ca: 4.9 mg/L) | |
| MgSO4∙7H2O | 1.5 g/L | 15 mg/L (S: 1.95 mg/L) | |
| KH2PO4 | 0.16 g/L | 1.6 mg/L (P: 0,36 mg/L) | |
| 2: Fe-EDTA | FeCl3∙6H2O | 64 mg/L | 64 µg/L (Fe: 13 µg/L) |
| Na2EDTA∙2H2O | 100 mg/L | 100 µg/L | |
| 3: Trace elements | H3BO3a | 185 mg/L | 185 µg/L (B: 32 µg/L) |
| MnCl2∙4H2O | 415 mg/L | 415 µg/L (Mn: 115 µg/L) | |
| ZnCl2 | 3 mg/L | 3 µg/L (Zn: 1.4 µg/L) | |
| CoCl2∙6H2O | 1.5 mg/L | 1.5 µg/L (Co: 0.37 µg/L) | |
| CuCl2∙2H2O | 0.01 mg/L | 0.01 µg/L (Cu: 3.7 ng/L) | |
| Na2MoO4∙2H2O | 7 mg/L | 7 µg/L (Mo: 2.8 µg/L) | |
| 4: NaHCO3 | NaHCO3 | 50 g/L | 50 mg/L (C: 7.14 mg/L) |
Table 1: Concentrations of nutrients in stock solutions for algal growth medium
NOTE: H3BO3 can be dissolved by adding 0.1 M NaOH. EDTA should be removed when testing metals, to avoid complexation with metal ions. Sterilize the stock solutions by membrane filtration (mean pore diameter 0.2 µm) or by autoclaving (120 °C, 15 min). Do no autoclave stock solutions 2 and 4, but sterilize them by membrane filtration. Store the solutions in the dark at 4 °C.
- To produce 1 L of algal growth medium, transfer 500 mL sterilized ultrapure water into a 1 L sterilized volumetric flask and add 10 mL of stock solution 1: Macronutrients, 1 mL of stock solution 2: Fe-EDTA, 1 mL of stock solution 3: Trace elements, and 1 mL of stock solution 4: NaHCO3.
- Fill up to 1 L with sterilized ultrapure water, stopper the flask and shake thoroughly to homogenize the algal growth medium.
- Equilibrate the solution before use by leaving it overnight in contact with air or by bubbling with sterile, filtered air for 30 min. After equilibration, adjust the pH, if necessary, to pH 8.1 ± 0.2, with either 1 M HCl or 1 M NaOH.
3. Setting up the algal test
NOTE: A flow diagram of the algal test procedure is shown in Figure 2.
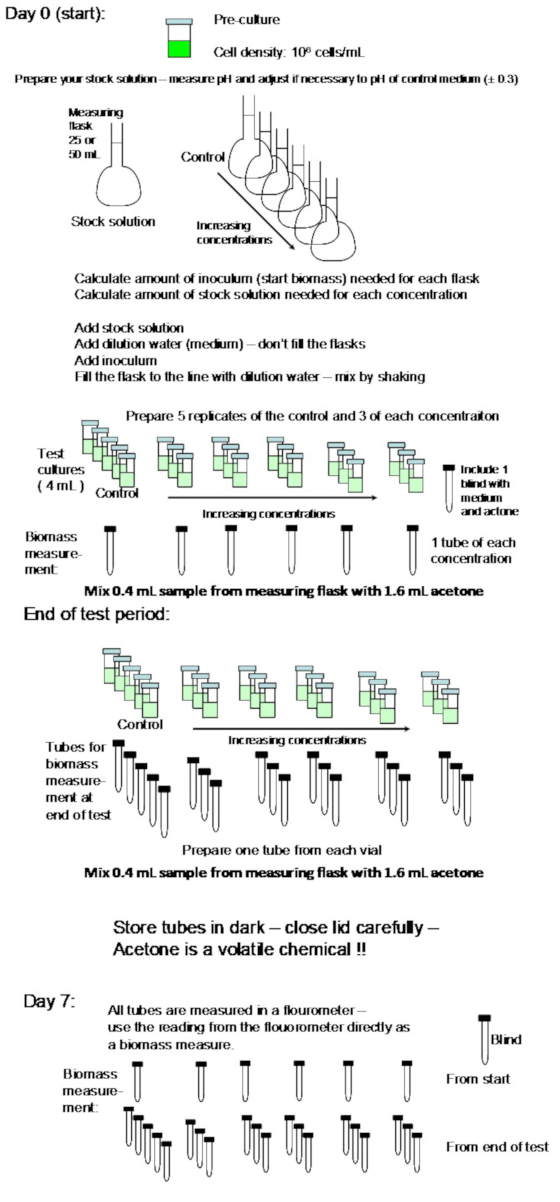
Figure 2: Flow diagram of the algal test setup. Please click here to view a larger version of this figure.
- Prepare a stock solution of the test compound at the desired highest test concentration in the algal growth medium prepared according to step 2. For preparation of stock solutions/suspensions, follow OECD 201 (for soluble compounds) or OECD 318 (for nanomaterials).
- Measure the pH in the stock solution. If it deviates more than one unit from the algal growth medium, adjust the pH to 8 with either 1 M HCl or 1 M NaOH.
- Calculate the inoculum volume needed to reach a final cell concentration of 1 x 104 cells/mL in a 25 mL test solution.
NOTE: The inoculum should come from a culture of uncontaminated exponentially growing Raphidocelis subcapitata grown using the LEVITATT setup. - Calculate the amount of stock solution to add to each 25 mL volumetric flask to obtain the desired test concentrations. The factor between each concentration should not exceed 3.2.
- Mark one 25 mL volumetric flask for each chosen concentration and an additional 25 mL volumetric flask marked control.
- Add the amount of stock solution of the test compound needed to reach the desired concentrations to the 25 mL volumetric flask. Do not add stock solution to the control.
- Add the medium to each 25 mL volumetric flask to reach a volume of approximately 20 mL.
- Add the volume of inoculum calculated in step 3.3 to each 25 mL volumetric flask. Add the medium to each 25 mL volumetric flask to a final total volume of 25 mL.
- Stopper the flasks and mix thoroughly by turning the flasks two times vertically.
- Transfer 0.4 mL from each flask into individual screw cap vials and add 1.6 mL of acetone (saturated with MgCO3): one sample for each test concentration and the control. Close the lids tightly and store in the dark at room temperature until fluorescence measurements (section 4).
- Pipet 4 mL of each test solution into 20 mL scintillation vials (3 replicates per concentration and 5 replicates for the control). Screw lids on the scintillation vials. Remember that the lids must have a drilled hole (approximately 1 mm in diameter) to allow for CO2 exchange.
- After 24 h, 48 h, and 72 h, pipet 0.4 mL from each vial into screw cap vials and add 1.6 mL of acetone (saturated with MgCO3). Close the lids tightly and store in the dark at room temperature until fluorescence measurements (section 4).
- After the last sample is taken at 72 h, gently pool the three replicates for a given concentration in one vial and measure the pH. Repeat for all concentrations and the control. The pH should not deviate more than 1.5 units from the initial pH for any of the samples measured.
- Discharge the remaining liquids into a waste container following your institutional rules and regulations.
4. Analyzing algal test samples
- Use a fluorescence spectrophotometer to measure the algal biomass (here expressed as chlorophyll A). The peak emission for chlorophyll A is 420 nm for the excitation wavelength and 671 nm for the emission wavelength.
- Measure the fluorescence of each individual sample three times and calculate the average value for each sample.
- Use equation 1 to calculate the growth rate. The measured fluorescence (relative units) can be used directly as the biomass parameter in equation 1.
Equation 1: µ = (ln Nt – ln N0) / t
where µ is the growth rate (d-1), N0 is the initial biomass, Nt is the biomass at time t, and t is the length of the test period (d). Note, N0 and Nt should be expressed in the same unit. - Use a statistical software to fit a non-linear regression curve (e.g., a log-logistic or Weibull function) to the growth rate data to obtain effective concentration values at 10%, 20%, and 50% inhibition. In the supplementary information an example of code for fitting in the statistical software R using the DRC package11 is given.
Wyniki
An initial test with a reference substance is carried out to determine the sensitivity of the algal strain. Reference substances regularly used for R. subcapitata are potassium dichromate and 3,5-Dichlorphenol7,8. Figure 3 and Table 2 show a representative result of an algal test including curve fitting and statistical outputs when the DRC package in R is applied to the growth rates.
Dyskusje
Phytoplankton converts solar energy and carbon dioxide to organic matter and thus holds a pivotal role in the aquatic ecosystem. For this reason, algal growth rate inhibition tests are included as one of three mandatory aquatic toxicity tests required for regulatory risk assessment of chemicals. The ability to perform a reliable and reproducible algal toxicity test is key in this regard. Test setups using Erlenmeyer flasks introduces a range of variabilities and inconveniences as described in the introduction. To circumv...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This research was funded by PATROLS – Advanced Tools for NanoSafety Testing, Grant agreement 760813 under Horizon 2020 research and innovation program.
Materiały
| Name | Company | Catalog Number | Comments |
| Acetone | Sigma-Aldrich | V179124 | |
| Ammonium chloride | Sigma-Aldrich | 254134 | |
| BlueCap bottles (1L) | Buch & Holm A/S | 9072335 | |
| Boric acid | Sigma-Aldrich | B0394 | |
| Calcium chloride dihydrate | Sigma-Aldrich | 208290 | |
| Clear acrylic sheet (40x40 cm) | |||
| Cobalt(II) chloride hexahydrate | Sigma-Aldrich | 255599 | |
| Copper(II) chloride dihydrate | Sigma-Aldrich | 307483 | |
| Ethylenediaminetetraacetic acid disodium salt dihydrate | Sigma-Aldrich | E5134 | |
| Fluorescence Spectrophotometer F-7000 | Hitachi | ||
| Hydrochloric acid | Sigma-Aldrich | 258148 | |
| Iron(III) chloride hexahydrate | Sigma-Aldrich | 236489 | |
| LED light source | Helmholt Elektronik A/S | H35161 | Neutral White, 6500K |
| Magnesium chloride hexahydrate | Sigma-Aldrich | M9272 | |
| Magnesium sulfate heptahydrate | Sigma-Aldrich | 230391 | |
| Manganese(II) chloride tetrahydrate | Sigma-Aldrich | 221279 | |
| Orbital shaker | IKA | 2980200 | |
| Potassium phosphate monobasic | Sigma-Aldrich | P0662 | |
| Raphidocelis subcapitata | NORCCA | NIVA-CHL1 strain | |
| Scintillation vials (20 mL) | Fisherscientific | 11526325 | |
| Sodium bicarbonate | Sigma-Aldrich | S6014 | |
| Sodium hydroxide | Sigma-Aldrich | 415413 | |
| Sodium molybdate dihydrate | Sigma-Aldrich | 331058 | |
| Spring clamp | Frederiksen Scientific A/S | 472002 | |
| Thermostatic cabinet | VWR | WTWA208450 | Alternative: temperature controlled room |
| Ventilation pipe (Ø125 mm) | Silvan | 22605630165 | |
| Volumetric flasks (25 mL) | DWK Life Sciences | 246781455 | |
| Zinc chloride | Sigma-Aldrich | 208086 |
Odniesienia
- European Chemicals Agency. Guidance on Registration. European Chemicals Agency. , (2016).
- Organisation for Economic Cooperation and Development. Guidance Document on Aquatic Toxicity Testing of Difficult Substances and Mixtures. Organisation for Economic Cooperation and Development. , (2019).
- Blaise, C., Legault, R., Bermingham, N., Van Coillie, R., Vasseur, P. A simple microplate algal assay technique for aquatic toxicity assessment. Toxicity Assessment. 1 (3), 261-281 (1986).
- Hjorth, R., Sorensen, S. N., Olsson, M. E., Baun, A., Hartmann, N. B. A certain shade of green: can algal pigments reveal shading effects of nanoparticles. Integrated Environmental Assessment and Management. 12 (1), 200-202 (2016).
- Chen, F., et al. Algae response to engineered nanoparticles: current understanding{,} mechanisms and implications. Environmental Science: Nano. 6 (4), 1026-1042 (2019).
- Arensberg, P., Hemmingsen, V. H., Nyholm, N. A miniscale algal toxicity test. Chemosphere. 30 (11), 2103-2115 (1995).
- Organisation for Economic Cooperation and Development. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test. Organisation for Economic Cooperation and Development. , (2011).
- International Organization for Standardization (ISO). Water Quality - Fresh Water Algal Growth Inhibition Test with Unicellular Green Algae. International Organization for Standardization (ISO). , (2012).
- Halling-Sørensen, B., Nyhohn, N., Baun, A. Algal toxicity tests with volatile and hazardous compounds in air-tight test flasks with CO2 enriched headspace. Chemosphere. 32 (8), 1513-1526 (1996).
- Mayer, P., Nyholm, N., Verbruggen, E. M. J., Hermens, J. L. M., Tolls, J. Algal growth inhibition test in filled, closed bottles for volatile and sorptive materials. Environmental Toxicology and Chemistry. 19 (10), 2551-2556 (2000).
- Ritz, C., Baty, F., Streibig, J. C., Gerhard, D. Dose-response analysis using R. PloS One. 10 (12), 0146021 (2015).
- Birch, H., Kramer, N. I., Mayer, P. Time-resolved freely dissolved concentrations of semivolatile and hydrophobic test chemicals in in vitro assays-measuring high losses and crossover by headspace solid-phase microextraction. Chemical Research in Toxicology. 32 (9), 1780-1790 (2019).
- Trac, L. N., Schmidt, S. N., Mayer, P. Headspace passive dosing of volatile hydrophobic chemicals - toxicity testing exactly at the saturation level. Chemosphere. 211, 694-700 (2018).
- Eisentraeger, A., Dott, W., Klein, J., Hahn, S. Comparative studies on algal toxicity testing using fluorometric microplate and Erlenmeyer flask growth-inhibition assays. Ecotoxicology and Environmental Safety. 54 (3), 346-354 (2003).
- Paixao, S. M., Silva, L., Fernandes, A., O'Rourke, K., Mendonca, E., Picado, A. Performance of a miniaturized algal bioassay in phytotoxicity screening. Ecotoxicology. 17 (3), 165-171 (2008).
- Thellen, C., Blaise, C., Roy, Y., Hickey, C. Round-robin testing with the selenastrum--capricornutum microplate toxicity assay. Hydrobiologia. 188, 259-268 (1989).
- Nagai, T., Taya, K., Annoh, H., Ishihara, S. Application of a fluorometric microplate algal toxicity assay for riverine periphytic algal species. Ecotoxicology and Environmental Safety. 94, 37-44 (2013).
- Lee, W. M., An, Y. J. Effects of zinc oxide and titanium dioxide nanoparticles on green algae under visible, UVA, and UVB irradiations: no evidence of enhanced algal toxicity under UV pre-irradiation. Chemosphere. 91 (4), 536-544 (2013).
- Samei, M., Sarrafzadeh, M. H., Faramarzi, M. A. The impact of morphology and size of zinc oxide nanoparticles on its toxicity to the freshwater microalga, Raphidocelis subcapitata. Environmental Science and Pollution Research. 26 (3), 2409-2420 (2019).
- Neale, P. A., Jaemting, A. K., O'Malley, E., Herrmann, J., Escher, B. I. Behaviour of titanium dioxide and zinc oxide nanoparticles in the presence of wastewater-derived organic matter and implications for algal toxicity. Environmental Science: Nano. 2 (1), 86-93 (2015).
- Hartmann, N. B., et al. The challenges of testing metal and metal oxide nanoparticles in algal bioassays: titanium dioxide and gold nanoparticles as case studies. Nanotoxicology. 7 (6), 1082-1094 (2013).
- Farkas, J., Booth, A. M. Are fluorescence-based chlorophyll quantification methods suitable for algae toxicity assessment of carbon nanomaterials. Nanotoxicology. 11 (4), 569-577 (2017).
- Handy, R. D., et al. Practical considerations for conducting ecotoxicity test methods with manufactured nanomaterials: what have we learnt so far. Ecotoxicology. 21 (4), 933-972 (2012).
- Handy, R. D., et al. Ecotoxicity test methods for engineered nanomaterials: practical experiences and recommendations from the bench. Environmental Toxicology and Chemistry. 31 (1), 15-31 (2012).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone