Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Macro-Rheology Characterization of Gill Raker Mucus in the Silver Carp, Hypophthalmichthys molitrix
W tym Artykule
Podsumowanie
This protocol presents a method to perform rheology characterization of mucus that resides on gill rakers (GRs) of the silver carp. Viscoelastic characteristics of GR-mucus, obtained by measuring viscosity, storage and loss moduli, are evaluated for the apparent yield stress to understand the filter feeding mechanism in GRs.
Streszczenie
The silver carp, Hypophthalmichthys molitrix, is an invasive planktivorous filter feeder fish that infested the natural waterways of the upper Mississippi River basin due to its highly efficient filter feeding mechanism. The characteristic organs called gill rakers (GRs), found in many such filter feeders, facilitate the efficient filtration of food particles such as phytoplankton that are of a few microns in size.
The motivation to investigate the rheology of the GR mucus stems from our desire to understand its role in aiding the filter feeding process in the silver carp. The mucus-rich fluid, in a ‘thick and sticky’ state may facilitate the adhesion of food particulates. The permeation and transport through the GR membrane are facilitated by the action of external shear forces that induce varying shear strain rates. Therefore, mucus rheology can provide a vital clue to the tremendous outcompeting nature of the silver carp within the pool of filter feeding fish. Based on this it was posited that GR mucus may provide an adhesive function to food particles and act as a transport vehicle to assist in the filter feeding process.
The main objective of the protocol is to determine the yield stress of the mucus, attributed to the minimum shear stress required to initiate flow at which irreversible plastic deformation is first observed across a structured viscoelastic material. Accordingly, rheological properties of the GR mucus, i.e., viscosity, storage, and loss moduli, were investigated for its non-Newtonian, shear-thinning nature using a rotational rheometer.
A protocol presented here is employed to analyze the rheological properties of mucus extracted from the gill rakers of a silver carp, fished at Hart Creek location of the Missouri River. The protocol aims to develop an effective strategy for rheological testing and material characterization of mucus assumed to be a structured viscoelastic material.
Wprowadzenie
The silver carp, Hypophthalmichthys molitrix, is a planktivorous filter feeder and an invasive species that has infiltrated several natural waterways in the United States. This species was initially introduced in the upper Mississippi River basin to control algal blooms1,2,3. The silver carp is an extremely efficient feeder. Typically, its consumable food particle sizes range from 4 to 20 μm to larger zooplankton that are around 80 μm3,4,5. This species has outcompeted other native fish and can potentially cause enormous damage to native waterways by limiting available resources1,2,6. Thus, filter feeding fish such as the silver carp and the bighead carp pose a major threat to the Great Lakes1,2,6,7,8.
Filter feeding fish possess special organs called the gill rakers (GRs) with a thin layer of mucus residing on their surface. These organs improve the efficiency of filtration and aggregation of small particles from the incoming fluid. The goal of the protocol presented herein is to characterize the non-Newtonian, shear thinning material property and yield stress of the GR mucus acquired from the inner surface of the gill rakers in the silver carp. The value of yield stress of the GR-mucus, ascertained using a rotational rheometer, is of interest in this study. The measured yield stress also called the “apparent yield stress” depends on the testing methods such as steady shear rate- or dynamic oscillatory strain-type9,10. The shear-thinning, ‘yield-stress fluid,’ undergoes a transition from solid-like to liquid-like behavior at a critical applied stress9,11. The apparent yield stress is the minimum shear stress required to initiate flow or that at which irreversible plastic deformation is first observed when the mucus transitions from a gel-like material to a fluid-like material. This behavior can be observed in structured viscoelastic materials. The transition from gel-like to fluid-like behavior of the GR mucus entails two functions i.e., an adhesive role to gather food particulates and a transport vehicle role to assist in the particulate delivery and filtration process. The extended function of the mucus includes creating diffusion barriers in disease resistance and respiration, providing controlled release of nutritional factors, toxic components and excretion, creating metabolic pathways for feeding and nesting, helping in predator protection, and producing boundary layer modifications that improve the locomotion and propulsive efficiency12,13,14.
Unlike simple fluids, complex fluids like the mucus possess properties that vary with flow conditions and require additional measurement parameters to define their bulk scale physical behavior. To monitor the viscosity and yield stress of GR mucus, rheological measurements are performed using a rotational rheometer. The rotational rheometer applies a steady or oscillatory shear stress or strain by means of a rotating disk in contact with the fluid sample and measures its response. The rationale behind using this instrument and technique is that the rheometer can provide a set of measurements to describe the material properties of the GR mucus of the silver carp, which cannot be defined by viscosity alone.
The mucus is a viscoelastic material and its mechanical response to an imposed deformation is between that of a pure solid (governed by Hooke’s law of elasticity) and that of a pure liquid (governed by Newton’s law of viscosity)15,16. The complex macromolecular network contained within the mucus can stretch and reorient in response to external forces or deformation. A rotational rheometer is comprised of a cone geometry and a Peltier plate as shown in Figure 1 and Figure 2 (see Table 1 for instrumentation specifications). The objective of this study was to develop a protocol to determine the rheological properties of the GR mucus. An advantage of the rotational rheometer over a viscometer is its ability to make dynamic measurements using small sample volumes. The GR mucus sample volume in this study was approximately 1.4 mL. The viscometer, on the other hand, is limited to constant shear rates and requires large sample volumes.
The rheological properties of the mucus are expected to vary greatly within the silver carp anatomy. For example, the properties of the mucus residing on the GR surfaces may be different from the epibranchial organ. To account for the potential variability of mucus properties in different regions of the fish, the acquired GR mucus sample was diluted, and solutions of three concentrations were created and tested using the rotational rheometer.. The data and results regarding mucus rheology reported after executing the protocol demonstrated the efficacy of the measurement technique. The illustrative data presented in this paper are not meant to be generalized across the entire silver carp population. The protocol presented herein can be extended to investigate mucus rheology across larger sample sets to test other hypotheses.
The purpose of this study is to demonstrate the variation of rheological properties of GR mucus rheology with three different mucus concentrations (400 mg/mL, 200 mg/mL and 100 mg/mL). The 400 mg/mL concentration represents the raw mucus sample harvested from the fish GRs. Deionized water (DI) was used to dilute the raw mucus sample into 200 mg/mL and 100 mg/mL concentrations. Diluting the mucus samples allowed for the evaluation of the degree of shear thinning and apparent yield stress as a function of concentration and the determination of the concentration at which the GR mucus transitions to non-Newtonian behavior. A shaker was used to break down any large clumps of mucus in the samples to mitigate errors in the rheological data due to inhomogeneity.
In most vertebrates, including fish, the predominant mucus-forming macromolecules are glycoproteins (mucins) that tend to swell in water by entanglements or chemical cross-linking and create a gel-like material12,13,17,18,19,20. The high-molecular-weight, gel-forming macromolecules and high-water content reflects the slipperiness in the mucus13. A high degree of inter-macromolecular interactions leads to gel-formation whereas lower levels of inter-macromolecular interactions or broken bonds result in high-viscosity fluids21.
The processes of food particulate filtration in filter feeding fish are aided by GR mucus-related properties such as cohesion and viscosity that determine its potential for adhesion and tack22. The strength of mucus-based adhesion depends on specific intermolecular, electrostatic or hydrophobic interactions23. Sanderson et al.24 conducted a suspension-feeding study in blackfish wherein they found the evidence for mucus-based adhesion. They stated that the adhesion of suspended food particulates with a mucosal surface is followed by the transport of aggregated clumps of particles bound together with mucus by directed water-flow acting on it24. The mucus exposed to shear strain rates generated from water-flow facilitates the delivery of food particulates to digestive organs. Endoscopic techniques were used to observe filtered particles24.
Literature on the range of shear rates and practical limits in the rheological testing of GR mucus is scarce. Therefore, guidance was sought from rheological studies on gastric, nasal, cervical and lung mucus, salmon skin mucus, hagfish slime, and bone-joint surface lubricant wherein the rheological characterization and non-Newtonian attributes were studied11,12,25,26,27,28,29,30,31. More recently, the effect of fish skin mucus on locomotion and propulsive efficiency has been studied using constant shear rate viscometry. Skin mucus rheology studies (without any dilution or homogenization) pertaining to seabream, sea bass and meagre demonstrated non-Newtonian behavior at typically low shear rates14. In another related study, the raw skin mucus samples from dorsal and ventral sides of the Senegalese sole were found to exhibit non-Newtonian behavior, indicating a higher viscosity of the ventral mucus at all shear rates considered32. Other rheological protocols pertaining to the hydrogel scaffold development and for highly concentrated suspensions using a constant shear rate viscometer have also been reported in the literature33,34.
In this study, the GR mucus properties were investigated using a strain rate controlled, rotational rheometer that has been widely used in rheology experiments on complex biological fluids25. For Newtonian fluids, the apparent viscosity remains constant, is shear-rate-independent and the shear stresses vary linearly with shear strain rates (Figure 3A, B). For non-Newtonian fluids (such as shear-thinning fluids) viscosity is shear-rate-dependent or deformation-history-dependent (Figure 3A, B). The loss modulus (G”) represents the extent to which the material resists the tendency to flow and is representative of fluid viscosity (Figure 4). The storage modulus (G’) represents the tendency of the material to recover its original shape following stress-induced deformation and is equivalent to elasticity (Figure 4). The phase angle (δ) or loss tangent value, is calculated from the inverse tangent of G”/G’. It represents the balance between energy loss and storage and is also a common parameter for characterizing viscoelastic materials (δ = 0° for a Hookean solid; δ = 90° for a viscous liquid; δ < 45° for a viscoelastic solid and δ > 45° for a viscoelastic liquid) (Figure 4)25. The apparent yield stress (σy) in structured fluids represents a change of state that can be observed in rheological data from steady state sweep and dynamic stress-strain sweeps10. If the external applied stress is less than the apparent yield stress, the material will deform elastically. When the stress exceeds the apparent yield stress (marked as “average stress” in Figure 3B), the material will transition from elastic to plastic deformation and begin to flow in its liquid state35. Measuring the storage modulus (G’) and loss modulus (G”) in the mucus-sample under oscillatory stress (or strain) conditions quantifies the change in the material state from gel-like to viscoelastic liquid-like behavior.
The types of rheometer tests performed to monitor data pertaining to storage modulus (G’), loss modulus (G”) and apparent viscosity (η) are described here. The dynamic oscillation tests (strain sweeps and frequency sweeps) monitored G’ and G” under controlled oscillation of cone geometry. The dynamic strain sweep tests determined the linear viscoelastic region (LVR) of the mucus by monitoring the intrinsic material response (Figure 4). Strain sweeps were used to determine the yielding behavior at constant oscillation frequency and temperature. The dynamic frequency sweep tests monitored the material response to increasing frequency (rate of deformation) at a constant amplitude (strain or stress) and temperature. Strain was maintained in the linear viscoelastic region (LVR) for the dynamic frequency sweep tests. The steady-state shear rate tests monitored the apparent viscosity (η) under steady rotation of the cone geometry. The GR mucus was subjected to incremental stress steps and apparent viscosity (η, Pa.s) was monitored for varying shear rate (ý, 1/s).
The protocol presented in this paper treats the GR mucus as a complex structured material of unknown viscoelasticity with a certain linear viscoelastic response range. The fish mucus was extracted from the GRs of the silver carp during a fishing expedition at the Hart creek location in the Missouri River by Professor L. Patricia Hernandez (Department of Biological Sciences, The George Washington University) 1,2,36. An array of GRs inside the mouth of a Silver carp is shown in Figure 5A and a schematic drawing is presented in Figure 5B. An excised GR is shown in Figure 5C. The extraction of mucus from GRs of the silver carp is presented as an example in the schematic drawings, Figure 5D, E. All the rheometer tests were performed under a constant, controlled temperature of 22 ± 0.002 °C, the temperature recorded at the fishing site1,2,36. Each mucus sample was tested three times with the rheometer, and the averaged results are presented along with the statistical error bars.
Protokół
1. Preparation of the mucus solutions of various concentrations
NOTE: Three concentrations of the mucus solution (400 mg/mL, 200 mg/mL and 100 mg/mL with approximate volumes, 1 mL, 1 mL, and 2 mL, respectively) are prepared for this experiment.
- To calculate the mass of the mucus, measure the average mass of the vials with (Mwith-mucus ; mg) and without mucus (Mvials ; mg). Then subtract the mass of the vials with mucus with that without mucus (Mmucus = Mwith-mucus - Mvials ; mg).
- Dilute the mucus into three concentrations (400, 200, 100, mg/mL) with deionized (DI) water.
- Prepare the first concentration of the mucus solution, 400 mg/mL by adding 0.6 mL DI water to the mucus using a micropipette.
NOTE: Since the approximate volume of the extracted mucus was 1.4 mL, the 400 mg/mL solution will have a total volume of ~ 2 mL. - Place the 400 mg/mL mucus solution vial on a shaker to make sure that the mucus solution is adequately homogenized, and any mucus particulate agglomeration is mitigated.
- Prepare the second concentration of the mucus solution, 200 mg/mL, by drawing half the volume of the first-concentration mucus solution into a new vial using a micropipette and adding 1 mL of DI water into the new vial.
- Repeat step 1.2.2 for the first and second vials with mucus solutions.
- Prepare the third concentration of the mucus solution, 100 mg/mL, by drawing half the volume (1 mL) of the 200 mg/mL solution into a new vial using a micropipette and add of DI water into the new vial.
- Repeat step 1.2.2 for all three concentrations of mucus solutions in their respective vials (see Supplementary Figure 1).
- Store the mucus solution vials in a refrigerator until the rheometer calibration and testing is performed.
- Prepare the first concentration of the mucus solution, 400 mg/mL by adding 0.6 mL DI water to the mucus using a micropipette.
2. Measurements and data acquisition using a rheometer
NOTE: The software used in this protocol for instrument control and data acquisition with rheometer are noted in the Table of Materials. This software will be referred as ‘rheometer instrument control software’.
- Set up and calibrate the rheometer instrument.
- Turn on the compressed air supply to the rheometer and make sure the pneumatic table and the rheometer are leveled using a bubble gauge. Twist off the protective cap on the rheometer shaft and hold shaft still whilst unscrewing.
- Turn on the rheometer main switches to activate the magnetic bearings on the rheometer.
- Turn on the rheometer control computer with the rheometer instrument control software installed in it and launch the rheometer instrument control software (see Supplementary Figure 2).
- Perform instrument calibration by selecting the tabs, 'Calibration | Instrument' from the software window. Choose 'Instrument' option. Click on 'Calibrate' under ‘Inertia’. Record the instrument inertia calibration value in μN.m.s2 and repeat calibration at least 3x to ensure calibration values are within 10% of each other (see Supplementary Figure 3).
- Install the rheometer geometry on the shaft of the rheometer.
- Click the ‘Geometries’ tab in the rheometer instrument control software.
- Clean the cone with the desired geometry, (40 mm diameter, 1◦ 0’ 11’’ cone) and Peltier plate with isopropanol (see Table 1, Table of Materials, Figure 1, and Figure 2).
NOTE: The Peltier Plate comes installed on the rheometer; it can be cleaned with isopropanol while it is directly fixed to the rheometer. - Ensure that the Peltier plate fixture is free from any visible dust and clean, if necessary, with isopropanol. Install the Peltier plate if it is not pre-installed in the rheometer and connect the heat sink connections.
- Press the ‘Lock button’ on rheometer to the lock shaft that is connected to the cone geometry. This arrests the position of the shaft, but it can rotate freely at the position.
- Click on ‘Smart Swap | Enabled’ in the rheometer instrument control software tab to allow automatic detection of the geometry (see Supplementary Figure 4).
- Turn the shaft on top of the rheometer to screw on geometry. The software will detect the 40 mm diameter, 1◦ 0’ 11’’ cone angle geometry at this stage (see Table 1 and Table of Materials).
- Repeat steps 2.2.5 – 2.2.6 to ensure that the geometry is detected.
- Select ‘Gap’ under the ‘Control Panel’ of the rheometer instrument control software, click on ‘Options’ icon and chose ‘Axial Force’ option. Set axial force to ‘1 Newton’; this is to ensure the cone geometry touches the Peltier plate for zero gap initialization (see Supplementary Figure 5).
- Perform the rheometer geometry calibration.
- Select the tab, 'Geometry' from the software window. Click on 'Calibrate' under ‘Inertia’. Record the geometry inertia calibration value in μN.m.s2 and repeat this 2-3 times to ensure calibration values are within 10% of each other.
- Click on 'Calibrate' under ‘Friction’ in the software window. Record the geometry friction calibration value in μN.m/(rad/s) and repeat this 2-3 times to ensure calibration values are within 10% of each other (see Supplementary Figure 6).
- Perform the zero-gap initialization
NOTE: Since the geometry cannot be accurately raised above the Peltier Plate to perform measurements without a reference “zero” position, zero-gap initialization is performed. For the measurement purposes, the geometry has a built-in geometry gap of 24 µm and a trim gap of 28 µm. The trim gap is set to effectively clean the excess fluid that may spill outside the surface area of the geometry. These gaps are imperative for accurately measuring data using the sample and the rheometer. The step 2.4.1 is absolutely required to make sure that the geometry is set to zero gap for achieving the geometry and trim gaps of 24 µm and 28 µm, respectively.- Click on the ‘Zero gap’ icon under ‘Gap’ tab in the ‘Control Panel’ in the software window. The initialization is complete when the axial force experienced by the geometry is greater than or equal to 1 N, as it touches the Peltier plate. Ensure that the rheometer gap is zeroed so that its reference position is accurate (see Supplementary Figure 7 and Supplementary Figure 8).
- Press ‘up and down arrow’ controls on the rheometer instrument or ‘geometry raise and lower’ icons under the ‘Gap’ tab in the rheometer instrument control software to raise the geometry to any arbitrary height. The control screen on the rheometer instrument and the control panel of the rheometer instrument control software will display the (same) gap height.
- Set up the experimental procedure in the rheometer instrument control software. Perform the characterization of rheological properties by using a cone-on-Peltier plate geometry at 22 °C.
NOTE: The US Geological Survey website was used to ascertain the river water temperature River on September 20, 2018, when the silver carp used for the GR mucus experiments were fished at the Hart Creek location36. The temperature of the mucus can affect the rheological properties. The significance of adjusting the values to river temperature is to approximately match the temperature under which the mucus properties can be realistically estimated.- Select the tab, 'Experiments' in the rheometer instrument control software and fill in the relevant information such as 'Name', 'Operator', 'Project' etc. (see Supplementary Figure 9)
- Select the tab, 'Geometry' and make sure the information agrees with steps 2.2.5. - 2.2.7. (see Supplementary Figure 10).
- Select the tab, 'Procedure', and use the arrow keys set up '1: Oscillation Amplitude' procedure. (see Supplementary Figure 11).
- Initialize 'Environmental Control' settings as the following: 'Temperature = 22 °C'; 'Soak Time = 120 s' and check the box 'Wait for Temperature' (see Supplementary Figure 11).
- Initialize 'Test Parameters' settings as the following: 'Frequency = 1 Hz'; set 'Logarithmic sweep'; 'Torque = 10 to 10000 μN.m'; 'Points per decade = 5' (see Supplementary Figure 11).
- Set up the experiment to determine the Linear Viscoelastic Range (LVR) of the mucus of known concentration (100 mg/mL)
- Using an appropriate micropipette and pipette tip draw approximately 0.3 mL of fish mucus solution of concentration 100 mg/mL (see Step 1.2, Table of Materials).
- Introduce the mucus solution on to the Peltier plate using the micropipette (see Figure 2).
- Press ‘Trim Gap’ button on rheometer to lower the geometry on to the Peltier plate. Alternatively, click on ‘Trim Gap’ icon under ‘Gap’ tab in the ‘Control Panel’ option in the rheometer instrument control software (see Supplementary Figure 12).
- Use the micropipette with the pipette tip to remove any excess mucus solution and ensure that the fluid is underneath the geometry without any spilling near the periphery of the geometry.
NOTE: Improper loading of the fluid will lead to errors in the measurements. Under filled sample will lower torque distribution and over filled sample will lead erroneous stress distributions due to spilling along the edges. - Select ‘Motor’ and ‘Velocity’ tabs to 5 rad/s and 0 rad/s alternately, until there is minimal inertia and velocity in the sample underneath the geometry. The control screen on the rheometer instrument and the control panel of the rheometer instrument control software will display the velocity (see Supplementary Figure 13).
- Press ‘Geometry Gap’ button on rheometer to lower geometry to the preset suitable gap per specific geometry. Alternatively, click on ‘Geometry Gap’ icon under ‘Gap’ tab in the ‘Control Panel’ option in the rheometer instrument control software (see Supplementary Figure 14).
- Run the experiment to determine the Linear Viscoelastic Range (LVR) of the mucus of the known concentration (100 mg/mL).
- Click ‘Start’ icon on the rheometer instrument control software (see Supplementary Figure 15).
NOTE: The rheometer performs automatic measurements; once the ‘Start’ button is pressed, the rheometer will take approximately 20 min to complete the test. The ‘Points per decade’ setting in Step 2.5.5 determines how much time the rheometer will need to complete measurements. - Run the experiment by clicking ‘Yes’ on the pop-up box that appears and suggests that the geometry gap be lowered to the correct distance to start the experiment, if not already lowered.
- Observe the real time plot generated by the rheometer that reports the storage (G’) and loss (G’’) moduli.
NOTE: The G’ and G” are the storage and loss moduli, respectively. The storage modulus represents the tendency for the material to recover its original shape following stress-induced deformation and is equivalent to elasticity. The loss modulus represents the extent to which the material resists the tendency to flow and is representative of fluid viscosity (see Figure 4). - Set the X-axis of the plot to ‘Oscillation strain percentage’. To do this, right click on the graph presented and choose the ‘Graph Variables’ tab (see Supplementary Figure 16).
- Record the oscillation strain percentage range from the plot before material enters the Non-Linear Viscoelastic range, once the test is complete.
- Press ‘up and down arrow’ controls on the rheometer instrument or ‘geometry raise and lower’ icons under the ‘Gap’ tab in the rheometer instrument control software to raise the geometry to any arbitrary height above the Peltier plate.
- Save the file that contains both the experimental procedure and results in the native file format of the rheometer instrument control software to ascertain the linear viscoelastic region (LVR) of the mucus sample.
NOTE: This can be done by setting the X-axis of the plot to strain amplitude (%) and/or oscillation stress before the data enters the non-linear viscoelastic region (NLVR) (see Supplementary Figure 16).
before the data enters the non-linear viscoelastic region (NLVR) (see Supplementary Figure 16).
- Click ‘Start’ icon on the rheometer instrument control software (see Supplementary Figure 15).
- Run the dynamic sweeps and steady state shear rate flow test experiments in Linear Viscoelastic Range (LVR) for the mucus of known concentration 100, mg/mL to generate results from three independent mucus samples of 100 mg/mL. Perform these steps on the available mucus concentration samples individually.
- Repeat steps 2.5.1 – 2.5.4.
- Initialize 'Test Parameters' settings as the following: 'Frequency = 1 Hz'; set 'Logarithmic sweep'; 'Strain % = 100 to 10000 %; 'Points per decade = 10'.
- Select the 'Procedure' tab and use the arrow keys set up '2: Oscillation Frequency' procedure.
- Initialize 'Environmental Control' settings as the following: 'Temperature = 22 °C'; 'Soak Time = 0.0 s'.
- Initialize 'Test Parameters' settings as the following: 'Strain % = 1 %'; set 'Logarithmic sweep'; 'Frequency = 20 to 1 Hz'; 'Points per decade = 10'.
- Select the 'Procedure' tab and use the arrow keys set up '3: Flow Sweep' procedure.
- Initialize 'Environmental Control' settings as the following: 'Temperature = 22 °C'; 'Soak Time = 0.0 s'.
- Initialize 'Test Parameters' settings as the following: 'Shear rate = 1 to 10000 1/s'; 'Points per decade = 10'; check box ‘Steady state sensing’.
- Repeat steps 2.7.1 – 2.7.2 and wait until the experiment is complete, approximately 45 minutes.
- Press ‘up and down arrow’ controls on the rheometer instrument or ‘geometry raise and lower’ icons under ‘Gap’ tab in the rheometer instrument control software to raise the geometry to any arbitrary height.
- Use disposable wipes and gloves to remove and clean the mucus on the Peltier plate with isopropanol solution (see Table of Materials).
- Save the file that contains both the experimental procedure and results in the native file format of the rheometer instrument control software.
3. Repeat the protocol for other concentrations of mucus solutions of 200 mg/mL and 400 mg/mL.
- Perform steps 2.5 – 2.8 including all the sub-steps listed therein for the remaining two concentrations of mucus solutions, 200 mg/mL and 400 mg/mL.
4. Graphical representation and data analysis
NOTE: The code provided in the supplemental code file performs data averaging and generates repeatability-errors, overlays the data from all experiments. The standard-deviation calculation features are not available in the rheometer instrument control software. The code is written in a programming language for data analysis, post-processing and graphical representation (see Table of Materials for details).
- Export data generated from step 2.8 pertaining to the 100 mg/mL GR mucus concentration and step 3.1 pertaining to the 200mg/mL and 400 mg/mL GR mucus concentrations into spreadsheet-format by clicking on the tab, ‘File | Export | Excel’ in the rheometer instrument control software (see Supplementary Figure 17).
- Run supplemental codes to generate plots of apparent viscosity (η) for varying shear strain rates (
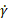 ) and loss modulus (G”), storage modulus (G’) and phase angle (δ) for varying oscillation stress (
) and loss modulus (G”), storage modulus (G’) and phase angle (δ) for varying oscillation stress ( ) and generate representative results.
) and generate representative results.
Wyniki
In this section, we present the results of the experiments on GR mucus using a rotational rheometer with a cone geometry (40 mm diameter, 1° 0’ 11’’) and a Peltier plate. The experiments helped in characterizing the non-Newtonian, shear-thinning behavior of the GR mucus and the apparent yield stress depicting the mucus transition from a gel-like material to a fluid-like material. The representative results entail quantitative descriptions of low-torque limits and secondary flow effects of the rotat...
Dyskusje
One of the main objectives of developing this protocol is to establish that it is well-suited for rheological characterization of GR mucus when very small sample volumes are available. We acknowledge that more samples from a school of silver carp are needed to fully characterize the rheological properties of the GR mucus and the data presented herein are not a generalization across the entire silver carp population. Our technique is justified because of its efficacy with rheological characterization of small sample volum...
Ujawnienia
No conflicts of interest declared.
Podziękowania
The authors acknowledge support and funding from the GW Center for Biomimetics and Bioinspired Engineering. We thank Professor L. Patricia Hernandez of the Department of Biological Sciences at The George Washington University for inspiring the investigation and ongoing collaboration, providing biological expertise on the physiology of the silver carp and providing the mucus samples. We thank the students, Mr. David Palumbo, Ms. Carly Cohen, Mr. Isaac Finberg, Mr. Dominick Petrosino, Mr. Alexis Renderos, Ms. Priscilla Varghese, Mr. Carter Tegen and Mr. Raghav Pajjur for help in the laboratory and Mr. Thomas Evans and Mr. James Thomas of TA Instruments, New Castle, DE for support with training and maintenance of the rheometer. Images for Figures 5A,C were taken during a dissection performed by Professor L. Patricia Hernandez of the Department of Biological Sciences at The George Washington University.
Materiały
| Name | Company | Catalog Number | Comments |
| Materials | |||
| Kim Wipes | VWR | 470224-038 | To clean Sample from plate |
| Gloves | VWR | 89428-750 | To prevent contamination of sample |
| Pipette | VWR | 89079-974 | To transport sample from vial to rheometer |
| Pipette Tips | Thermo Scientific | 72830-042 | To transport sample from vial to rheometer |
| Shaker | VWR | 89032-094 | To homogenously mix sample of mucus |
| Vials | VWR | 66008-710 | Contains measured sample volumes |
| Weigh Scale | Ohaus | Scout –SPX Balances | To weigh mass of mucus samples |
| Chemical Reagents | |||
| De-Ionized Water (H20) | - | - | Liquid |
| Sterile 70% Isopropanol (C3H8O) | VWR | 89108-162 | Liquid |
| GR Mucus | |||
| 100 mg/mL concentration, 2mL | - | - | Viscoelastic Material |
| 400 mg/mL concentration, 1mL | - | - | Viscoelastic Material |
| 200 mg/mL concentration, 1mL | - | - | Viscoelastic Material |
| Software | |||
| MATLAB | Mathworks | R2017a | Data analysis, post-processing and graphical representation |
| Trios | TA Instruments | v4.5.042498 | Rheometer instrument control and analysis software |
Odniesienia
- Cohen, K. E., Hernandez, L. P. The complex trophic anatomy of silver carp, Hypophthalmichthys molitrix, highlighting a novel type of epibranchial organ. Journal of Morphology. 279, 1615-1628 (2018).
- Cohen, K. E., Hernandez, L. P. Making a master filterer: Ontogeny of specialized filtering plates in silver carp (Hypophthalmichthys molitrix). Journal of Morphology. 279, 925-935 (2018).
- Cremer, M., Smitherman, R. Food habits and growth of silver and bighead carp in cages and ponds. Aquaculture. 20 (1), 57-64 (1980).
- Battonyai, I., et al. Relationship between gill raker morphology and feeding habits of hybrid bigheaded carps (Hypophthalmichthys spp.). Knowledge and Management of Aquatic Ecosystems. 416, 36 (2015).
- Zhou, Q., Xie, P., Xu, J., Ke, Z., Guo, L. Growth and food availability of silver and bighead carps: Evidence from stable isotope and gut content analysis. Aquaculture Research. 40 (14), 1616-1625 (2009).
- Freedman, J. A., Butler, S. E., Wahl, D. H. . Impacts of invasive Asian carps on native food webs (Final Report). , (2012).
- Nico, L., Fuller, P., Li, J. . Silver carp (Hypophthalmichthys molitrix)-FactSheet. , (2017).
- Walleser, L., Howard, D., Sandheinrich, M., Gaikowski, M., Amberg, J. Confocal microscopy as a useful approach to describe gill rakers of Asian species of carp and native filter-feeding fishes of the upper Mississippi River system. Journal of Fish Biology. 85 (5), 1777-1784 (2014).
- Nelson, A. Z., Ewoldt, R. H. Design of yield-stress fluids: a rheology-to-structure inverse problem. Soft Matter. 13, 7578-7594 (2017).
- Chen, T. Rheological Techniques for Yield Stress Analysis. TA Instruments Applications Note, RH025. , (2020).
- Ewoldt, R. H., Johnston, M. T., Caretta, L. M., Spagnolie, S. Experimental challenges of shear rheology: how to avoid bad data. Complex Fluids in Biological Systems. , (2015).
- Thornton, D. J., Sheehan, J. K. From Mucins to Mucus: Toward a more coherent understanding of this essential barrier. Proceedings of the American Thoracic Society. 1, 54-61 (2004).
- Shepard, K. L. Functions for fish mucus. Reviews in Fish Biology and Fisheries. 4, 401-429 (1994).
- Fernández-Alacid, L., et al. Skin mucus metabolites in response to physiological challenges: A valuable non-invasive method to study teleost marine species. Science of the Total Environment. 644, 1323-1335 (2018).
- Wagner, C. E., Wheeler, K. M., Ribbeck, K. Mucins and Their Role in Shaping the Functions of Mucus Barriers. Annual Reviews in Cell and Developmental Biology. 34, 189-215 (2018).
- Bird, R. B., Armstrong, R. C., Hassager, O. . Dynamics of Polymeric Liquids, Volume 1: Fluid Mechanics. , 1255-1284 (1987).
- Mantle, M., Allen, A. Isolation and characterisation of the native glycoprotein from pig small intestinal mucus. Biochemical Journal. 195, 267-275 (1981).
- Allen, A., Hutton, D. A., Pearson, J. P., Sellers, L. A., Nugent, J., O'Conner, M. Mucus glycoprotein structure, gel formation and gastrointestinal mucus function. Mucus and Mucosa (Ciba Foundation Symposium). , 137-156 (1984).
- Asakawa, M. Histochemical studies of the mucus on the epidermis of eel, Anguillajaponica. Bulletin of Japanese Society of Scientific Fisheries. 36, 83-87 (1970).
- Fletcher, T. C., Jones, R., Reid, L. Identification of glycoproteins in goblet cells of epidermis and gill of plaice (Pleuroneces platessa L.), flounder (Platichthys flesus (L.)) and rainbow trout (Salmo gairdneri Richardson). Histochemical Journal. 8, 597-608 (1976).
- Silberberg, A. Mucus glycoprotein, its biophysical and gel forming properties. Symposia of the Society for Experimental Biology. 43, 43-64 (1989).
- Hills, B. . The Biology of Surfactants. , 408 (1988).
- Aubert, H., Brook, A. J., Shephard, K. L. Measurement of the adhesion of a desmid to a substrate. British Phycology Journal. 24, 293-295 (1989).
- Sanderson, S. L., Cech, J. J., Patterson, M. R. Fluid dynamics in suspension feeding black fish. Science. 251, 1346-1348 (1991).
- Lai, S. K., Wang, Y. Y., Wirtz, D., Hanes, J. Micro- and macrorheology of mucus. Advanced Drug Delivery Reviews. 61 (2), 86-100 (2009).
- Chaudhary, G., Ewoldt, R. H., Thiffeault, J. L. Unravelling hagfish slime. Journal of Royal Society Interface. 16 (150), 20180710 (2019).
- Downing, S., Salo, W., Spitzer, R., Koch, E. The hagfish slime gland: a model system for studying the biology of mucus. Science. 214, 1143-1145 (1981).
- Hwang, S. H., Litt, M., Forsman, W. C. Rheological properties of mucus. Rheologica Acta. 8, 438-448 (1969).
- Litt, M. Mucus rheology. Archives of Internal Medicine. 126, 417-423 (1970).
- Quarishi, M. S., Jones, N. S., Mason, J. The rheology of nasal mucus: a review. Clinical Otolaryngology. 23, 403-413 (1998).
- Nordgård, C. T., Draget, K. I., Seternes, T. Rheology of salmon skin mucus. Annual Transactions - The Nordic Rheology Society. 23, 175-179 (2015).
- Fernández-Alacid, L., et al. Comparison between properties of dorsal and ventral skin mucus in Senegalese sole: Response to an acute stress. Aquaculture. 513, 734410 (2019).
- Yüce, C., Willenbacher, N. Challenges in Rheological Characterization of Highly Concentrated Suspensions - Case Study for Screen-printing Silver Pastes. Journal of Visualized Experiments. (122), e55377 (2017).
- Sultan, S., Mathew, A. P. 3D Printed Porous Cellulose Nanocomposite Hydrogel Scaffolds. J. Vis. Exp. (146), (2019).
- Barnes, H. A., Hutton, J. F., Walters, K. . An Introduction to Rheology. , (1989).
- USGS Current Conditions for USGS 06910450 Missouri River at Jefferson City, MO. U.S. Geological Survey Available from: https://nwis.waterdata.usgs.gov/usa/nwis/uv/?cb_00010=on&cb_00060=on&cb_00065=on&format=gif_default&site_no=0691045&p09-19&end_date=2018-09-21 (2020)
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone