Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Generation and Quantitative Characterization of Functional and Polarized Biliary Epithelial Cysts
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
Three-dimensional (3D) cellular systems are relevant models for investigating organogenesis. A hydrogel-based method for biliary cysts production and their characterization is proposed. This protocol unravels the barriers of 3D characterization, with a straightforward and reliable method to assess cyst formation efficiency, sizes, and to test their functionality.
Streszczenie
Cholangiocytes, the epithelial cells that line up the bile ducts in the liver, oversee bile formation and modification. In the last twenty years, in the context of liver diseases, 3-dimensional (3D) models based on cholangiocytes have emerged such as cysts, spheroids, or tube-like structures to mimic tissue topology for organogenesis, disease modeling, and drug screening studies. These structures have been mainly obtained by embedding cholangiocytes in a hydrogel. The main purpose was to study self-organization by addressing epithelial polarity, functional, and morphological properties. However, very few studies focus on cyst formation efficiency. When this is the case, the efficiency is often quantified from images of a single plane. Functional assays and structural analysis are performed without representing the potential heterogeneity of cyst distribution arising from hydrogel polymerization heterogeneities and side effects. Therefore, the quantitative analysis, when done, cannot be used for comparison from one article to another. Moreover, this methodology does not allow comparisons of 3D growth potential of different matrices and cell types. Additionally, there is no mention of the experimental troubleshooting for immunostaining cysts. In this article, we provide a reliable and universal method to show that the initial cell distribution is related to the heterogeneous vertical distribution of cyst formation. Cholangiocyte cells embedded in hydrogel are followed with Z-stacks analysis along the hydrogel depth over the time course of 10 days. With this method, a robust kinetics of cyst formation efficiency and growth is obtained. We also present methods to evaluate cyst polarity and secretory function. Finally, additional tips for optimizing immunostaining protocols are provided in order to limit cyst collapse for imaging. This approach can be applied to other 3D cell culture studies, thus opening the possibilities to compare one system to another.
Wprowadzenie
In the last three decades, the field of in vitro research has advanced towards 3D culture systems. A number of protocols have emerged for culturing cells in 3D as spheroids or aggregates in the presence or absence of a scaffold/matrix, in a drop, in agitation, in microfluidic devices, or floating1. The use of 3D culture methods has proved its advantages over 2-dimensional (2D) cultures, particularly for epithelial cells, which were shown to self-organize in 3D structures, called cysts or acini. In this case, the cells form a monolayer encircling a lumen, where cells acquire their full epithelial phenotype with improved physiological specific functions2.
Numerous studies have contributed to the development of methods for forming these epithelial organoids in natural matrices. This has allowed to recapitulate in vivo cell-cell and cell-microenvironment interactions, to get the establishment and the stability of the epithelial phenotype3,4,5,6,7. Recently, and in particular with the aim of developing transplantable organoids and deciphering the requirement of the microenvironment for orchestrating the epithelial program, synthetic hydrogels have been developed to enhance the formation of epithelial acini8,9,10. Unfortunately, these studies report on qualitative data, or present calculation methods using internal references such as the ratio of cysts over non-cysts in a 2D plane8,9,10. This precludes any comparison between different studies in terms of efficiency, stability, or morphological and physiological characterization of the epithelial organoids.
Microencapsulation of epithelial cells in beads using microfluidic devices has allowed for more realistic quantitative and comparative results. Using this technology, organoids from various cell types were formed and differentiated based on the morphology among different 3D cellular structures11,12. However, this technology is not easy to work with and requires the use of clean rooms to produce the microfluidic devices. This technology has been established for a few types of hydrogels but requires technical adaptation to be applied to other hydrogels, restricting its versatility. Therefore, most studies intended to develop epithelial organoids rely on the embedding of epithelial cells in a hydrogel bulk. In these methods, the high heterogeneity of gel structuration and cell distribution inside the whole 3D culture is often neglected. Therefore, most of the analyses relate to single 2D images, which represent only very roughly the distribution of the various cellular objects in the whole 3D volume.
Diseases that affect bile ducts, such as cholangiocarcinoma, biliary atresia, primary sclerosing cholangitis, among others, are a major cause of mortality and morbidity. Except for liver transplantation, there are no effective treatments for these conditions13. Efforts to investigate bile duct formation, disease causes, and progression will allow the development of novel therapies14.
Biliary organotypic models of cysts, spheroids or tube-like structures using normal or patient-derived, differentiated, or progenitor-derived cholangiocyte cell lines have been developed15,16,17,18,19,20. Various studies have recapitulated cholangiocyte polarity, expression of cholangiocyte markers, presence of cilia, cholangiocyte secretory and reabsorptive ability, and lumen formation and obstruction; all of which represent important characteristics of cholangiocyte phenotype, morphology, and function15,17,19. Others have reported maintenance of patient-derived biliary organoids for long periods of time20. Recently, we investigated the role of biochemical and biophysical cues on biliary cysts organogenesis21. Importantly, the pathogenesis of biliary atresia was studied in biliary spheroids and tubes7,22. Furthermore, key features of primary sclerosing cholangitis such as cholangiocyte senescence, secretion of pro-inflammatory cytokines, as well as macrophage recruitment were successfully studied using biliary spheroids15,20. However, reproducible in vitro 3D quantitative models that physiologically modulate cholangiocyte phenotype, physiology, and microenvironment where these questions can be addressed are still needed. Moreover, only few publications have reported cyst formation efficiency21,23. This is an important point to establish, particularly when investigating organogenesis, disease cause, and correlation of drug responses with cholangiocyte function and polarization. In addition, with differences in scaffold/matrix used from protocol to protocol, it is difficult to compare between systems. To solve these issues, we propose a quantitative, reliable, and universal method to generate biliary cysts mimicking lumen formation, cholangiocyte polarization, and cholangiocyte secretory function. Importantly, we present a systematic analysis performed along the Z-axis across the 3D gel when evaluating over time, cyst formation efficiency, size, viability, polarization, and functionality. Furthermore, we used a natural hydrogel and normal rat cholangiocytes (NRC)s, as an example for the protocol, but other natural or synthetic hydrogels, as well as epithelial cells could be used for the formation of 3D cystic structures.
Access restricted. Please log in or start a trial to view this content.
Protokół
1. Generation of cysts
NOTE: This protocol can be performed with any type of hydrogel, if the gelation allows embedding of cells.
- Hydrogel coating
NOTE: Proper hydrogel coating of the chamber slide is a critical step to avoid the formation of 2D cell layers on the bottom of the well, that might interfere with subsequent cyst imaging and impair the calculation of cyst formation efficiency.- To ensure homogeneity of the gel solution, thaw the hydrogel at 4 °C overnight (O/N).
- Precool pipette tips on ice or O/N at -20 °C and an 8-well chamber slide at -20 °C O/N.
- Place the hydrogel and the 8-well chamber slide on an ice bucket filled with ice.
- In a 15 mL conical tube, prepare a solution containing 40% hydrogel (V/V) in cold NRC complete medium (see Table of Materials) and place the tube on ice.
- To coat a chamber slide, using cold pipette tips, add 50 μL of hydrogel solution on the center of each well, and spread over the whole surface using a pipette tip, while holding the chamber slide on ice (Figure 1A).
NOTE: Spread the hydrogel solution as evenly as possible avoiding bubbles. - To polymerize the hydrogel, incubate the chamber slide for at least 15 min at 37 °C, 5% CO2.
- Cell preparation
- Warm up NRC complete medium, phosphate buffered saline (PBS), and trypsin-ethylenediamine tetraacetic acid (trypsin-EDTA) in a water bath pre-heated to 37 °C.
- While the hydrogel is polymerizing, ensure that NRCs are grown to 70% confluency in a T-25 cm2 collagen-coated flask21. Wash the cells once with pre-heated 1x PBS.
- Incubate the NRCs with 5 mL of pre-heated 1x PBS (for a T-25 cm2 flask) for 20 min at 37 °C, 5% CO2.
NOTE: This step, which shortens the incubation time with trypsin-EDTA is instrumental in retaining the self-organizing properties of cells. - Discard the PBS, add 1 mL of trypsin-EDTA, and incubate for 5-10 min at 37 °C, 5% CO2.
- Neutralize with 4 mL of pre-heated complete NRC medium. Collect and transfer the cell suspension into a 15 mL conical tube, and spin at 150 x g for 4 min.
- Discard the medium and resuspend the cell pellet in 5 mL of pre-heated medium.
- Using a 40 μm cell strainer, filter the cell solution into a 50 mL conical tube and count the cells.
NOTE: Passing the cells through a strainer is a critical step for the quantitative results to be reproducible i.e., to get almost similar size of cell aggregates to be embedded.
- Embedding of cell suspension in hydrogel solution
- Prepare a solution of 1,600 µL of 80% hydrogel (V/V) in cold complete NRC medium (tube 1); keep in ice. Dilute 5 x 105 cells/mL in 1,600 µL of cold complete NRC medium (tube 2) and keep in ice.
NOTE: This step must be performed quickly to avoid polymerization of the hydrogel while mixing it with the cell suspension and to maintain cell viability. - To prepare a cell seeding solution of 2.5 x 105 cells/mL in 40% hydrogel (V/V), mix tube 1 and tube 2. Add 400 μL of the cell solution into each well of the hydrogel-coated chamber slide avoiding bubbles (Figure 1B).
- Keep the chamber slide in an incubator at 37 °C with 5% CO2 until media change.
- After 2 days in culture, remove 250 μL of the medium from a corner of each well, be careful to not pipette out the hydrogel. Then, slowly add 250 μL of the culture medium. Change the medium every 2 days.
NOTE: Minimize the movement of the chamber slide, particularly during the cyst initiation.
- Prepare a solution of 1,600 µL of 80% hydrogel (V/V) in cold complete NRC medium (tube 1); keep in ice. Dilute 5 x 105 cells/mL in 1,600 µL of cold complete NRC medium (tube 2) and keep in ice.
2. Cyst quantification
- Cyst imaging
NOTE: This section should be performed quickly to not compromise the cell viability if the microscope is not equipped with a heating chamber to control CO2 and temperature. In order to ensure consistent quantification, representative of the cyst distribution in the full hydrogel volume, cysts are imaged via phase-contrast microscopy and serial imaging (Z-stacks), with pre-defined parameters throughout different time points.- Take a Z-stack along the depth of the hydrogel for each time point (Figure 1C, D). In this example, Z-stacks are taken at days 1, 2, 4, 7, and 10.
NOTE: Check that the initial cell distribution is uniform in the hydrogel to ensure the applicability of this method.- With a phase-contrast microscope equipped with an image acquisition software, select the 10x objective magnification in the manual nose-piece pad window (Figure 2B(1)).
- Switch on the white lamp and select the brightfield imaging option.
- Switch the camera on by selecting the “Play” button in the bar submenu. Focus on a field of cysts and set the exposure time (Figure 2B(2)). Open the Auto Capture Folder window for an automatic saving of images (Figure 2B(3)).
- Open the capture Z-series window and define with the Z screw the top and bottom planes of the Z-stack (same XY coordinates but different Z screened). Adjust the Z-step depending on the objective, the level of resolution and press the button “Run now” to launch the acquisition (Figure 2B(4)).
NOTE: In this example, cysts are spread over a hydrogel thickness of 520 µm. 26 images are acquired along the hydrogel depth with a 20 µm Z-step interval. Depending on the objective, the Z-step should be adjusted to not miss any cyst and to ensure the detection of single cells and aggregates. - Take at least 3 non-overlapping Z-stacks per well.
NOTE: This sampling is necessary when, like in this example, cysts are more numerous in the depth of the gel than on the edges due to heterogeneities in the hydrogel polymerization. - In order to have a representative dataset repeat step 2.1.1.5. for 3 wells in total.
NOTE: The heterogeneous distribution of cysts depends on the type of hydrogel, its polymerization, and the cell line. Considering three Z-stacks per well and three wells per experiment, a minimum of 200 cysts are imaged over nine Z-stacks to characterize cyst formation and cyst growth at each time point.
- Take a Z-stack along the depth of the hydrogel for each time point (Figure 1C, D). In this example, Z-stacks are taken at days 1, 2, 4, 7, and 10.
- Image processing
NOTE: In a hydrogel, NRCs can be found as single cells, cysts or aggregates. Cysts are identified by the presence of a round and thin contrasted cell shell enclosing a lumen, while cell aggregates present an irregular shape and do not have a lumen. Aggregates and single cells have a dense and contrasted appearance (Figure 3B(4)).- Open the Fiji software, open the Z-stack and go to the Fiji menu and click File | Open (Supplementary Figure 1). Select the Z-stack to analyze. If needed, select “Virtual Stack” option and click “Yes” for opening (Figure 3A(1)).
- Duplicate the stack via Image | Duplicate. Click on the box “Duplicate stack” and click “OK” (Supplementary Figure 2).
NOTE: In this example, Z-stacks are in .nd2 file format encoded in 16 bits. - Create a minimum intensity projection from the duplicated stack. Go to the Image menu | Stacks | Z Project. Select Projection type “Min Intensity” and click “OK” (Figure 3A(2)) (Supplementary Figure 3).
- Subtract the background from the projection. Go to the Process menu | Subtract Background. Type 500.0 pixels of rolling ball radius and click “light background” to render cysts more contrasted than the background (Figure 3A(3)) (Supplementary Figure 4).
NOTE: The rolling ball radius defines the size of the region on which background subtraction is operated. This parameter must be set to the size of the largest object to identify. - If contrast enhancement is needed, go to Image menu | Adjust | Brightness/Contrast | Auto | Apply. Fiji will automatically optimize brightness and contrast. In (Figure 3A(3)), the lower and upper gray values were set to 49702 and 65452, respectively (Supplementary Figure 5).
NOTE: If the projection is not calibrated, go to the Analyze menu | Set scale and type the corresponding calibration µm/pixel ratio (Supplementary Figure 6).
- Cyst counting and cyst size measurements
- To measure the approximate cyst diameter, select the Straight-line tool in the Fiji menu and draw a line across the diameter of each cyst on the final projection (Figure 3B(4)). Add the new region of interest (ROI) created for each cyst to the ROI manager: press the “t” shortcut on the keyboard for faster counting and opening of the ROI manager. Click “Show All” to see the counted cysts (Supplementary Figure 7)
- Check that no cyst has been left without counting by superimposing the set ROIs from the projection on the Z-stack. To do so, click on the Z-stack window to select it. In the ROI Manager, click “Show All” and move the cursor along the Z-stack to check that image per image, all cysts have been counted (Supplementary Figure 8).
- Once new cysts have been counted and ROIs added on step 2.3.1., select the ROI set and save it via the ROI Manager window by clicking More | Save (Supplementary Figure 9).
- Select all ROIs in the ROI Manager and click “Measure” in the ROI Manager to get the size of each cyst. This will open a new window of measurements named “Results” numbering each cyst and its estimated size. Then save in .csv format by clicking on the “Results” window and via the menu: File | Save As (Supplementary Figure 10).
NOTE: A macro can be created to semi-automatically process stacks, estimate cyst number/sizes from the projections, and store the data for faster counting procedure. To do so, select the tool “Record” in the bar menu, by clicking Plugins | Macros | Record.
- Quantification of cyst formation efficiency
- Count the number of cysts at day Y,
 on a projection (Y=1, 2, 4, 7 or 10).
on a projection (Y=1, 2, 4, 7 or 10). - To calculate the cyst formation efficiency for 1000 cells at day Y, divide the number of cysts counted at that time point by the number of cells seeded at day 0 inferred from the hydrogel volume and multiply by 1000 (Figure 3C, Figure 4).
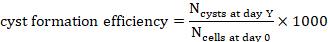
- Count the number of cysts at day Y,
3. Cell viability
- Prepare a stock solution of fluorescein diacetate (FDA) at 5 mg/mL by dissolving 5 mg of FDA in 1 mL acetone and store at -20 °C.
- Prepare a stock solution of propidium iodide (PI) at a concentration of 2 mg/mL in deionized water (dH2O) and store at 4 °C.
- Prepare NRC medium without fetal calf serum (FCS).
- To prepare the FDA/PI staining solution, add 4 μL of FDA stock solution (8 µg/mL final concentration) and 25 μL of PI stock solution (20 µg/mL final concentration) into 2.5 mL of NRC medium without FCS.
- Remove the medium from the chamber slide, add 250 μL of staining solution into each well and incubate 4-5 min in the dark at 37 °C, 5% CO2. Pipette out the staining solution carefully and wash once with 250 μL of 1x PBS.
- Carefully add 250 μL of complete NRC medium to each well and take pictures using an inverted fluorescence microscope with Texas red and fluorescein isothiocyanate (FITC) filters. Live cells will be green and dead cells will be red (Figure 5A).
NOTE: In order to quantify live/dead cells, take Z-stacks across the hydrogel volume following step 2 and adapt the image processing method for fluorescence.
4. Secretion activity
NOTE: The secretion activity through the apical membrane of the cholangiocytes is assessed by the secretion of fluorescein in the lumen. Its specificity can be evaluated by doing the same test with Verapamil, a multi-drug resistant (MDR) transporter inhibitor24.
- To prepare a staining solution of Hoechst 33258 at 5 µg/mL, add 0.83 μL of Hoechst stock solution (15 mg/mL stock concentration in dH2O) in 2.5 mL of NRC medium without FCS.
- Add 250 μL of Hoechst solution in each well and incubate at 37 °C, 5% CO2 for 15 min.
- Remove the Hoechst solution and add 250 µL of FDA solution (8 µg/mL final concentration) in each well. Incubate 4-5 min at 37 °C, 5% CO2.
NOTE: As soon as cells are exposed to FDA staining solution, the follow up of fluorescein secretion kinetics might be useful to calibrate the time needed for cysts to secrete. To do so, take pictures every min for 1 h via time-lapse imaging. In this example, the time needed to observe NRC secreting cysts in the hydrogel is about 15-20 min. - Take images using an inverted fluorescence microscope 5 min after rinsing with medium without FCS. Use 4′,6-diamidino-2-phenylindole (DAPI) and FITC filters to reveal nuclei labeling and fluorescein accumulation in the lumen (Figure 6A). To quantify the number of secreting cysts, take Z-stacks as in step 2 and adapt the image processing steps to fluorescent images.
NOTE: For the Verapamil test, precede the previous process (steps 4.3. to 4.4.) by an incubation with Verapamil, according to the following conditions: - Prepare a stock solution of 10 mM Verapamil in dimethyl sulfoxide (DMSO). To prepare 10 µM working solution, mix 2.5 μL of Verapamil stock solution with 2.5 mL culture medium without FCS.
- To assess that the fluorescence in the lumen results from MDR secretion, take another slide and add 250 µL of Verapamil working solution in each well and incubate 20 min at 37° C, 5% CO2
- Remove the solution and add 250 µL of FDA solution (8 µg/mL final concentration) into each well. Incubate 4-5 min in the dark at 37 °C, 5% CO2. Then, wash with 250 μL of 1x PBS, before imaging (Figure 6B, C).
5. Epithelial polarity assessment by immunofluorescence
- To prepare the fixing solution, mix 4% formaldehyde with 5% sucrose, in 1x PBS, pH 7.4 and incubate in a water bath pre-heated at 37 °C.
- To fix the cells, gently pipette out the culture medium from the well without damaging the matrix. Slowly add 400 µL of the fixing solution to the side of the wells. Incubate for 20 min at room temperature (RT).
NOTE: Always leave 25 µL of the liquid above the matrix to prevent its damage. - Gently remove the fixing solution and wash 3x with 400 μL of 1x PBS at (RT).
- Pipette out the PBS, add 400 µL of permeabilization solution (0.5% Triton X-100 in 1x PBS) and incubate 10 min at RT.
- Gently remove the permeabilization solution, followed by 3 quick washes with 400 μL of 1x PBS and a long washing step of 30 min at RT.
NOTE: At this step, the slide can be stored at 4 °C for 2 days. In this case, seal the slide with a paraffin film to prevent evaporation and matrix drying. - Remove the PBS, add 400 µL of blocking solution containing 0.1% bovine serum albumin (BSA) and 10% goat serum in 1x PBS and incubate for 60 min at RT.
CAUTION: Concentrations of BSA higher than 0.1% will result in lumen retraction and further cyst collapse (see Representative Results section) (Figure 7A). - Pipette out the blocking solution and wash once with 400 µL of PBS/0.05% Tween 20 and discard.
- Add 150 µL of the antibody solution, e.g., E-cadherin antibody diluted 1:400 and Phalloidin 568 (16.2 nM final concentration) in 1x PBS and incubate for 90 min at RT.
NOTE: This dilution of E-cadherin is the same used as in a standard 2D immunofluorescence protocol. - Wash the sample with 400 µL of PBS/0.05% Tween 20, 3x; each time incubating the sample for 10 min at RT.
- Add 150 µL of the secondary antibody (goat anti-rabbit IgG Alexa Fluor Plus 647), diluted 1:500 in 1x PBS and incubate 60 min at RT.
- Wash 3x with 400 µL of PBS/0.05% Tween 20, each time incubating the sample for 10 min at RT.
- Wash 3x with 400 µL of 1x PBS, each time incubating the sample for 10 min at RT.
- Discard the PBS of the last wash and prepare the chamber slide for visualization via confocal microscopy following one of the two options below.
- Add 400 μL of 1x PBS and 50 μL of DAPI per well. The samples can be examined through the bottom of the well without the need of mounting with a coverslip (Figure 7B).
- Add 100 µL per well of antifade reagent containing DAPI and let the slide drying O/N at RT.
Access restricted. Please log in or start a trial to view this content.
Wyniki
Formation and characterization of cysts
3D cell culture systems are an important tool to study organogenesis and disease modeling25. Unfortunately, most of these methods are qualitative or use internal quantification performed on a single plane by comparing the number of cysts versus non-cysts, in variable and often unspecified volumes, preventing any comparison in terms of cyst formation efficiency between the various studies7,
Access restricted. Please log in or start a trial to view this content.
Dyskusje
In order to study organogenesis and maintenance of 3D cellular structures, various tissues have been modelled, using different cellular origins but also different types of extra-cellular matrices including synthetic hydrogels8,9,10,21. However, due to lack of 3D quantitative analysis that allows for comparisons between methods in terms of organoids formation or functionality7<...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors have nothing to disclose.
Podziękowania
We thank Dr. Nicholas LaRusso (Mayo Clinic, Rochester, Minnesota, United States), who kindly provided the NRC cell line.
This work received the financial support of both the iLite RHU program (grant ANR-16-RHUS-0005) and the DHU Hepatinov.
We thank Isabelle Garcin and Réseau d’Imagerie Cellulaire Paris Saclay for their support on imaging.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| 10 µl- Pipette Eppendorf Research Plus | Thermo Fisher Scientific | 3120000020 | |
| 100 µl - Pipette Eppendorf Research Plus | Thermo Fisher Scientific | 3120000046 | |
| 1000 µl - Pipette Eppendorf Research Plus | Thermo Fisher Scientific | 3120000062 | |
| 1X PBS | Thermo Fisher Scientific | 14190-094 | |
| 200 µl - Pipette Eppendorf Research Plus | Thermo Fisher Scientific | 3120000054 | |
| 3,3′,5-Triiodo-L-thyronine sodium salt | Sigma-Aldrich | T5516 | NRC complete medium final concentration = 3.4 µg/mL |
| Acetic acid | VWR | 20104-298 | 0.02N final |
| Aerosol barrier pipettes tips 10 µl (Fisherbrand) | Thermo Fisher Scientific | 2707439 | |
| Aerosol barrier pipettes tips 1000 µl (Fisherbrand) | Thermo Fisher Scientific | 2707404 | |
| Aerosol barrier pipettes tips 200 µl (Fisherbrand) | Thermo Fisher Scientific | 2707430 | |
| Antibiotic Antimicotic Solution (100X) | Sigma-Aldrich | A5955 | NRC complete medium final concentration = 1:100 dilution |
| Bovine pituitary extract | Thermo Fisher Scientific | 13028-014 | NRC complete medium final concentration = 30 µg/mL |
| Bovine serum albumin | Sigma-Aldrich | A2153 | 1:1000 dilution |
| Chemically Defined Lipid Concentrate (100X) | Thermo Fisher Scientific | 11905-031 | NRC complete medium final concentration = 1:100 dilution |
| Collagen high concentration, rat tail | Thermo Fisher Scientific | 354249 | 50 µg/mL final concentration |
| Dexamethasone | Sigma-Aldrich | D4902 | NRC complete medium final concentration = 0.393 µg/mL |
| DMEM F12 | Thermo Fisher Scientific | 21331-020 | NRC complete medium final concentration = 1X |
| E-cadherin Rabbit anti-Human, Rat, Polyclonal | Thermo Fisher Scientific | PA5-32178 | 1:400 dilution |
| Eclipse TE300 inverted microscope | Nikon | imaging | |
| Ethanolamine | Sigma-Aldrich | E9508 | NRC complete medium final concentration = 0.32 mM |
| Fetal calf serum | Thermo Fisher Scientific | 10270-106 | NRC complete medium final concentration = 5:100 dilution |
| Fluoroshield with DAPI (Mounting medium) | Sigma-Aldrich | F6057 | |
| Formaldehyde 16% (W/V) | Thermo Fisher Scientific | 28906 | 4% (W/V) |
| Goat serum | Thermo Fisher Scientific | 16210-064 | 1:10 dilution |
| Hamamatsu camera (Digital camera C11440 ORCA - flash 4.OLT) | Hamamatsu | imaging | |
| Hoechst 33258 | Sigma-Aldrich | B1155 | 5 µg/mL final concentration |
| IgG (H+L) Highly Cross-Adsorbed Goat anti-Rabbit, Alexa Fluor Plus 647 | Thermo Fisher Scientific | A32733 | 1:500 dilution |
| ImageJ version 2.0.0-rc-69/1.52n | Open source image processing software | ||
| Insulin-Transferrin-Selenium (100X) | Thermo Fisher Scientific | 51300-044 | NRC complete medium final concentration = 1:100 dilution |
| L-Glutamine (100X) | Thermo Fisher Scientific | 25030-024 | NRC complete medium final concentration = 1:100 dilution |
| Matrigel GFR (stock concentration 9.7 mg/mL) | Thermo Fisher Scientific | 356231 | 4:10 dilution |
| NIS Elements software version 4.50.00 | Nikon | image acquisition and display | |
| Non-Essential-Amino-Acids-Solution (100X) | Thermo Fisher Scientific | 11140-035 | NRC complete medium final concentration = 1:100 dilution |
| Objective Plan Fluor 10X/0.30 Ph1 DL (∞/1.2 WD 15.2) | Nikon | ||
| Prolong Gold Antifade Reagent | Thermo Fisher Scientific | P36931 | |
| Propidium Iodide (PI) | Sigma-Aldrich | P4170 | 20 µg/mL final concentration |
| Rhodamine Phalloidin | Thermo Fisher Scientific | R415 | 16.2 nM final concentration |
| Sir-Actin / Verapamil kit | Spirochrome | SC001 | 10 µM final concentration |
| Soybean trypsin inhibitor | Thermo Fisher Scientific | 17075-029 | NRC complete medium final concentration = 50 µg/mL |
| Sterile cell strainer 40 µm (Fisherbrand) | Thermo Fisher Scientific | 22363547 | |
| Sterile pipettes 10 mL (Fisherbrand) | Thermo Fisher Scientific | 1367811E | |
| Sterile pipettes 5 mL (Fisherbrand) | Thermo Fisher Scientific | 1367811D | |
| Sterile tubes 1.5 mL (Fisherbrand) | Thermo Fisher Scientific | 11926955 | |
| Sterile tubes 15 mL (Fisherbrand) | Thermo Fisher Scientific | 7200886 | |
| Sterile tubes 50 mL (Fisherbrand) | Thermo Fisher Scientific | 553913 | |
| Sucrose | Sigma-Aldrich | S0389 | 5:100 dilution |
| Tissue culture treated flask 25cm2 (Falcon) | Thermo Fisher Scientific | 353108 | |
| Triton X-100 | Sigma-Aldrich | T8787 | 5:1000 dilution |
| Trypsin-EDTA (0.05%) phenol red | Thermo Fisher Scientific | 25300-054 | 1X |
| Tween-20 | Sigma-Aldrich | P1379 | 5:10000 dilution |
| Vitamin (100X) | Thermo Fisher Scientific | 11120-037 | NRC complete medium final concentration = 1:100 dilution |
| μ-Slide 8 Well ibiTreat, Ibidi | Clinisciences | 80826 |
Odniesienia
- Edmondson, R., Broglie, J. J., Adcock, F., Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. ASSAY and Drug Development Technologies. 12 (4), 207-218 (2014).
- Martín-Belmonte, F., et al. Cell-polarity dynamics controls the mechanism of lumen formation in epithelial morphogenesis. Current Biology. 18, 507-513 (2008).
- Debnath, J., Muthuswamy, S. K., Brugge, J. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 30 (3), 256-268 (2003).
- Artym, V. V., Matsumoto, K. Imaging Cells in Three-Dimensional Collagen Matrix. Current Procotols in Cell Biology. , Chapter 10 (Unit 10) (2010).
- Petersen, O. W., Ronnov-Jessen, L., Howlett, A. R., Bisell, M. J. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proceedings of the National Academy of Sciences of the United States of America. 89, 9064-9068 (1992).
- Kim, S. P., Lee, D. H., Park, J. K. Development of hepatocyte spheroids immobilization technique using alternative encapsulation method. Biotechnology and Bioprocess Engineering. 3, 96-102 (1998).
- Lorent, K., et al. Identification of a plant isoflavonoid that causes biliary atresia. Science Translational Medicine. 7 (286), 67(2015).
- Nowak, M., Freudenberga, U., Tsurkana, M. V., Wernera, C., Levental, K. R. Modular GAG-matrices to promote mammary epithelial morphogenesis in vitro. Biomaterials. 112, 20-30 (2017).
- Miroshnikova, Y. A., et al. Engineering Strategies to Recapitulate Epithelial Morphogenesis Within Synthetic Three-Dimensional Extracellular Matrix With Tunable Mechanical Properties. Physical Biology. 8 (2), 026013(2011).
- Ozdemir, T., et al. Tuning Hydrogel Properties to Promote the Assembly of Salivary Gland Spheroids in 3D. ACS Biomaterials Science & Engineering. 2 (12), 2217-2230 (2016).
- Dolega, M. E., Abeille, F., Picollet-D'hahan, N., Gidrol, X. Controlled 3D culture in Matrigel microbeads to analyze clonal acinar development. Biomaterials. 52, 347-357 (2015).
- Laperrousaz, B., et al. Direct transfection of clonal organoids in Matrigel microbeads: a promising approach toward organoid-based genetic screens. Nucleic Acids Research. 46 (12), 70(2018).
- Lazaridis, K. N., LaRusso, N. F. The Cholangiopathies. Mayo Clinic Proceedings. 90 (6), 791-800 (2015).
- Tam, P. K., Yiua, R. S., Lendahl, U., Andersson, E. R. Cholangiopathies - Towards a molecular understanding. EBioMedicine. 35, 381-393 (2018).
- Loarca, L., et al. Development and characterization of cholangioids from normal and diseased human cholangiocytes as an in vitro model to study primary sclerosing cholangitis. Laboratory Investigation. 97, 1385-1396 (2017).
- De Assuncao, T. M., Jalan-Sakrikar, N., Huebert, R. C. Regenerative medicine and the biliary tree. Seminars in Liver Disease. 37, 17-27 (2017).
- Dianat, N. H., et al. Generation of functional cholangiocyte-like cells from human pluripotent stem cells and HepaRG cells. Hepatology. 60, 700-714 (2014).
- Masyuk, A. I., et al. Cholangiocyte autophagy contributes to hepatic cystogenesis in polycystic liver disease and represents a potential therapeutic target. Hepatology. 67 (3), 1088-1108 (2018).
- Sampaziotis, F., Cardoso, M., Madrigal, P., Bertero, A., Saeb-Parsy, K., et al. Cholangiocytes derived from human induced pluripotent stem cells for disease modeling and drug validation. Nature Biotechnology. 33 (8), 845-852 (2015).
- Soroka, J. C., et al. Bile-Derived Organoids From Patients With Primary Sclerosing Cholangitis Recapitulate Their Inflammatory Immune Profile. Hepatology. 70 (3), 871-882 (2019).
- Funfak, F., et al. Biophysical Control of Bile Duct Epithelial Morphogenesis in Natural and Synthetic Scaffolds. Frontiers in Bioengineering and Biotechnology. 7 (417), 417(2019).
- Du, Y., et al. Bile Duct-on-a-Chip With Organ-Level Functions. Hepatology. 0 (0), (2019).
- Shiota, J. M., Mohamad Zaki, N. H., Merchant, J. L., Samuelson, L. C., Razumilava, N. Generation of Organoids from Mouse Extrahepatic Bile Ducts. Journal of Visualized Experiments. (146), e59544(2019).
- Bircsak, K. M., Richardson, J. R., Aleksunes, L. M. Inhibition of Human MDR1 and BCRP Transporter ATPase Activity by Organochlorine and Pyrethroid Insecticides. Journal of Biochemical and Molecular Toxicology. 27 (2), 157-164 (2013).
- Fennema, E., Rivron, N., Rouwkema, J., Blitterswijk, C., Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends in Biotechnology. 31 (2), 108-115 (2013).
- Kanade, S., Nataraj, G., Ubale, M., Mehta, P. Fluorescein Diacetate Vital Staining for Detecting Viability of Acid-Fast Bacilli in Patients on Antituberculosis Treatment. International Journal of Mycobacteriology. 5 (3), 294-298 (2016).
- Rieger, A. M., Nelson, K. L., Konowalchuk, J. D., Barreda, D. R. Modified Annexin V/Propidium Iodide Apoptosis Assay For Accurate Assessment of Cell Death. Journal of Visualized Experiments. (50), e2597(2011).
- Tabibian, J. H., Masyuk, A., Masyuk, T. V., O'Hara, S. P., LaRusso, N. F. Physiology of Cholangiocytes. Comprehensive Physiology. 3 (1), (2013).
- Spirlì, C., et al. Functional polarity of Na+/H+ and Cl-/HCO3- exchangers in a rat cholangiocyte cell line. American Journal Physiology. 275, 1236-1245 (1998).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone