Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Isolation of Proximal Fluids to Investigate the Tumor Microenvironment of Pancreatic Adenocarcinoma
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
Pancreatic juice is a precious source of biomarkers for human pancreatic cancer. We describe here a method for intraoperative collection procedure. To overcome the challenge of adopting this procedure in murine models, we suggest an alternative sample, tumor interstitial fluid, and describe here two protocols for its isolation.
Streszczenie
Pancreatic adenocarcinoma (PDAC) is the fourth leading cause of cancer-related death, and soon to become the second. There is an urgent need of variables associated to specific pancreatic pathologies to help preoperative differential diagnosis and patient profiling. Pancreatic juice is a relatively unexplored body fluid, which, due to its close proximity to the tumor site, reflects changes in the surrounding tissue. Here we describe in detail the intraoperative collection procedure. Unfortunately, translating pancreatic juice collection to murine models of PDAC, to perform mechanistic studies, is technically very challenging. Tumor interstitial fluid (TIF) is the extracellular fluid, outside blood and plasma, which bathes tumor and stromal cells. Similarly to pancreatic juice, for its property to collect and concentrate molecules that are found diluted in plasma, TIF can be exploited as an indicator of microenvironmental alterations and as a valuable source of disease-associated biomarkers. Since TIF is not readily accessible, various techniques have been proposed for its isolation. We describe here two simple and technically undemanding methods for its isolation: tissue centrifugation and tissue elution.
Wprowadzenie
Pancreatic ductal adenocarcinoma (PDAC) is one of the most aggressive tumors, and soon to become the second leading cause of death1,2,3. It is well-known for its immunosuppressive microenvironment and for its unresponsiveness to immunotherapy protocols4. Currently, surgical resection is still the only curative option for PDAC, yet there is a high frequency of early relapses and postsurgical complications. The lack of specific symptoms until an advanced stage does not allow for an early diagnosis, contributing to the deadliness of the disease. Furthermore, the overlap of symptoms between PDAC and other benign pancreatic pathologies can hamper the achievement of a prompt and reliable diagnosis with the current diagnostic strategies. The identification of variables associated to specific pancreatic pathologies could facilitate the surgical decision-making process and improve patient profiling.
Promising results in biomarker discovery have been achieved using easily accessible body fluids, such as blood5,6,7, urine8, saliva9 and pancreatic juice10,11,12. Many studies have exploited comprehensive “omics” approaches, such as genomic, proteomic and metabolomic techniques, to identify candidate molecules or signatures that could discriminate between PDAC and other benign pancreatic afflictions. We recently demonstrated that pancreatic juice, a relatively unexplored body fluid, can be used to identify metabolic signatures of patients with distinct clinical profiles12. Pancreatic juice is a protein-rich fluid, which accumulates the secretome of pancreatic ductal cells and flows to the main pancreatic duct, and then to the main common bile duct. Due to its proximity to the pancreas, it could be strongly affected by microenvironmental perturbations induced by the tumor mass (Figure 1), and therefore more informative than blood or urine, or tissue-based profiling. Several studies have explored the potential of pancreatic juice to identify novel biomarkers of disease using various approaches, including cytologic analysis13, proteomic analysis performed by mass-spectrometry14,15, assessment of genetic and epigenetic markers such as K-ras and p53 mutations16,17, alterations in DNA methylation18, and miRNAs19. Technically, pancreatic juice can be collected intraoperatively or with minimally invasive procedures, such as endoscopic ultrasound, retrograde cholangio-pancreatography, or by endoscopic collection of duodenal juice secretion20. It is not yet clear to what extent pancreatic juice composition is affected by the collection technique used. We describe here the intraoperative collection procedure and show that pancreatic juice can represent a precious source for PDAC biomarkers.
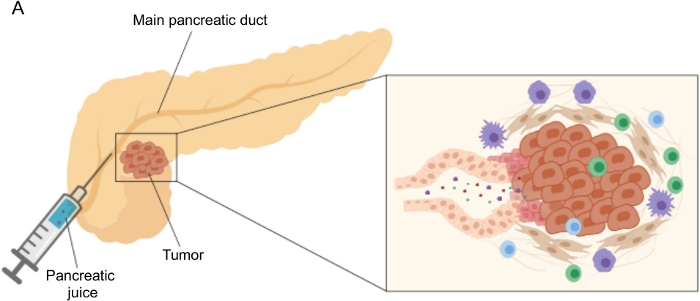
Figure 1: Schematic representation of pancreatic juice collection. (A) Schematic representation depicting the secretion of pancreatic juice into the pancreatic duct and its collection during surgery. The inset shows a close-up of the tumor microenvironment: pancreatic juice collects molecules released by tumor and stromal cells in the pancreatic ducts. Please click here to view a larger version of this figure.
The collection of pancreatic juice in genetic and orthotopic mouse models of PDAC would be appreciated in the perspective to exploit this biofluid in preclinical mechanistic studies; however, this procedure can be technically very challenging and is not feasible for simpler models such as subcutaneous tumors. For this reason, we identified tumor interstitial fluid (TIF) as an alternative source to pancreatic juice, for its similar characteristic of acting as an indicator of surrounding perturbations. Interstitial fluid (IF) is the extracellular liquid, found outside blood and lymphatic vessels, which bathes tissue cells21. IF composition is affected by both blood circulation to the organ and local secretion; in fact, surrounding cells actively produce and secrete proteins in the IF21. The interstitium reflects microenvironmental changes of surrounding tissues and could therefore represent a valuable source for biomarker discovery in several pathological contexts, such as tumors. The high concentration of locally secreted proteins in TIF can be used to identify candidate molecules to be tested as prognostic or diagnostic biomarkers in plasma22,23,24. Several studies have proven TIF to be a suitable sample for high-throughput proteomic approaches, such as mass spectrometry techniques23,24,25, as well as multiplex ELISA approaches26, and microRNA profiling27.
Several approaches have been proposed for the isolation of IF in tumors, which can be broadly categorized as in vivo (capillary ultrafiltration28,29,30,31 and microdialysis32,33,34,35) and ex vivo methods (tissue centrifugation22,36,37,38 and tissue elution39,40,41,42). These techniques have been reviewed in extensive detail43,44. The choice of the appropriate method should take into account issues such as the downstream analyses and applications and the volume recovered. We recently used this approach as a proof of principle to demonstrate the different metabolic activity of tumors from two murine pancreatic adenocarcinoma cell lines12. Based on literature24,38, we chose to use the low speed centrifugation method to avoid cell breakage and dilution from intracellular content. Both the amount of glucose and lactate in TIF reflected the different glycolytic characteristics of the two different cell lines. Here we describe in detail the protocol for the two most commonly used methods for the isolation of TIF: tissue centrifugation and tissue elution (Figure 2).
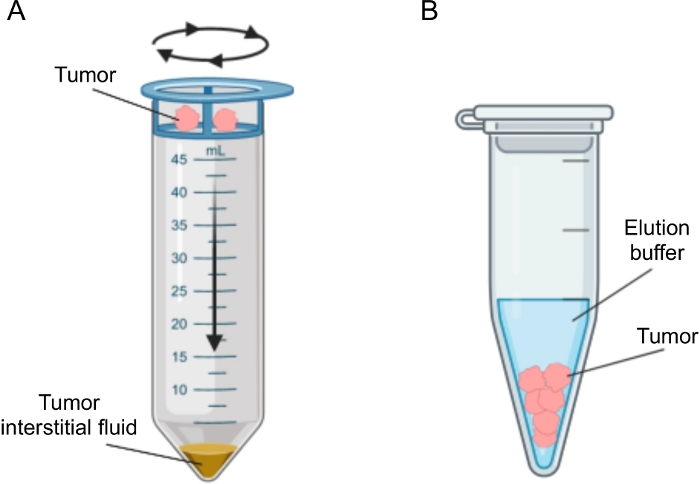
Figure 2: Schematic representation of tumor interstitial fluid isolation methods. Schematic illustration of the techniques described in detail in the protocol, namely tissue centrifugation (A) and tissue elution (B). Please click here to view a larger version of this figure.
Protokół
For all patients enrolled, peripheral blood and pancreatic juice were collected at the time of surgery according to protocols approved by the Ethical Committee of the Institution. All the patients were enrolled in the study after signed informed consent including collection of biological specimens and clinical data. The study was approved by the Ethical Committee of the Institution (protocol number ICH-595, approval issued on May 2009). Procedures involving mice and their care were conformed to EU and Institutional Guidelines (protocol ID 121/2016-PR).
1. Isolation of pancreatic juice
NOTE: The withdrawal of pancreatic juice is executed in the context of an open procedure of pancreatic resection (e.g., pancreaticoduodenectomy, total pancreatectomy, distal pancreatectomy) by an equipe of expert pancreatic surgeons.
- Patient selection
- Consider for the procedure any patient scheduled for an open pancreatic resection.
- Confirm inclusion if main pancreatic duct size is deemed sufficient to allow pancreatic juice retrieval. The minimum limit in diameter of the main pancreatic duct is considered 2 mm at contrast-enhanced CT imaging.
- Pre-operative study of pancreatic duct at contrast-enhanced CT imaging to help the planning of pancreatic juice retrieval
- Tridimensionally localize the main pancreatic duct within the gland at the level of the pancreatic neck: measure the distance of the main pancreatic duct from the anterior, superior and inferior pancreatic margin on cross-sectional slides, and coronal and sagittal renderings. Once in the operating theatre, use these measurements to approximate the correct place where to puncture the pancreas to cannulate the Wirsung duct and to retrieve pancreatic juice.
- Preparation of material
- Sterile material: Open the sterile envelope of one 25 G needle and one 3 mL syringe, and position them in the sterile field with the cooperation of the scrub nurse.
- Unsterile material: Keep a 3 mL K2EDTA vacuum test tube ready at hand in the operating room for the storage of the fluid.
- Preparation of the patient
- Position the patient on the operating room bed. Induce mixed general anesthesia, using Remifentanil, Sevorane and Rocuronium, then intubate and start ventilating the patient. Position the patient in supine decubitus with the right arm tucked to the body and the left arm abducted to 90° degrees secured on an armboard.
- Disinfect the skin of the abdomen at the site of the incision. Create and maintain a sterile field on the abdomen draping the patient.
- Surgical procedure
- Perform a subcostal incision and gain access to the abdominal cavity. Position a Rochard abdominal retraction for organ exposure.
- Expose and mobilize the pancreas through Kocher maneuver, opening of the gastrocolic ligament, incision of the retroperitoneal tissue along superior and inferior border of the pancreas creating a dissection plane between pancreatic neck and superior mesenteric vein located posteriorly.
- Once the pancreas is mobilized and exposed, proceed to pancreatic juice withdrawal before sectioning of pancreatic neck.
- Identification and localization of the pancreatic duct
- Estimate the location of the pancreatic duct using the measurement taken at imaging and then palpate the anterior surface of the pancreas to identify its precise location.
- Collection of pancreatic juice
- Hold the pancreatic head and the duodenum from underneath and elevate it with the left hand, marking the location of the pancreatic duct with the first digit.
- Take hold with the right hand of the 3 mL syringe with the 25 G needle mounted on.
- Use the right hand to insert the needle in the pancreas just distal to the left thumb. Decide the depth of penetration and the degree of inclination of the needle based on preoperative measurements and on the perception of having penetrated the duct wall.
- Withdraw the juice with the syringe. If it is not possible to retrieve the juice, relocate the needle in the four directions trying to cannulate the pancreatic duct.
- Once the pancreatic juice is retrieved, move it outside the sterile field and transfer it to the 3 mL K2EDTA vacuum test tube. Keep at 4 °C until the sample is transferred to the lab and proceed to further processing as early as possible.
NOTE: The volume of pancreatic juice that can be recovered with this procedure varies greatly, ranging approximately from 0.2 mL to 3 mL in our experience. The amount of juice retrieved is highly dependent on the patient: the dimension of the Wirsung duct and the functional status of the pancreas (functioning versus atrophic gland). In our experience there is no expedient that can be used to increase the amount of pancreatic juice retrieved.
2. Processing of pancreatic juice
- Centrifuge pancreatic juice at 400 x g for 10 min at 4 °C to remove any cells or debris.
NOTE: Pancreatic juice should be clear and transparent in color before centrifugation. Blood contamination during surgery can sometimes occur, making the sample appear murkier and redder in color. Consider excluding such samples from further analyses. - Recover the supernatant, aliquot and store at -80 °C until further analyses.
3. Induction of subcutaneous tumors
NOTE: The murine Panc02 and DT6606 cell lines were obtained from Prof. Lorenzo Piemonti (San Raffaele Diabetes Institute, Milan, Italy) and Prof. Francesco Novelli (Center for Experimental Research and Medical Studies, Torino, Italy) respectively, as previously described12.
- Growth of tumor cells
- Culture Panc02 and DT6606 cells in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% fetal bovine serum (FBS), 2mM L-Glutamine and 1% penicillin-streptomycin antibiotic.
- Thaw frozen cells 1-2 weeks before tumor injection, according to the growth rate of the cell line.
- Grow cells at 37 °C with 5% CO2 and 95% humidity in sterile conditions.
- Detach cells with 0.025% Trypsin/EDTA solution for 5 min at 37 °C when they reach 80% confluency and eliminate trypsin by centrifugation.
NOTE: DT6606 are primary, not immortalized, cells, derived from the LSL-KrasG12D-Pdx1-Cre mouse, and should not be passaged more than 3 times before injection in vivo in order to maintain their original characteristics. It is recommended to thaw DT6606 cells 7-10 days prior to injection.
- Injection of tumor cells in vivo
- Trypsinize cells (see step 3.1.4) and wash them once with Phosphate-Buffered Saline (PBS). Eliminate PBS by centrifugation and resuspend cells in fresh PBS before counting.
- Count the cells and resuspend them in PBS at a concentration of 0.5-1 x 107 cells/mL in order to have a final concentration of 0.5-1 x 106 cells/100 μL to inject in each mouse. Prepare the cells in excess. Keep the cells at 4 °C or on ice until the end of the procedure.
- Group the animals (8-week old female C57BL/6J mice) in different cages according to different cell lines or treatments.
- Restrain the animals manually and anaesthetize them using a mixture of Ketamine (80 mg/kg) and Xylazine (10 mg/kg) or according to locally-approved procedures.
- Shave the site of injection, usually a flank above a leg, with an electric shaver and carefully clean the site of injection with alcohol.
- Pipette up and down the cell suspension with a 1 mL syringe, removing any air bubbles by moving the piston up and down. Attach a 25 G needle to the syringe and push the piston upwards until the cell suspension reaches the needle opening.
- Pinch the skin of the flank with flat-tipped forceps and carefully insert the needle at the base of skin fold between the forceps without puncturing the peritoneal cavity or the musculature. To verify the correct position of the needle, gently try to move the tip of the needle sideways under the skin. The needle should move freely.
- Slowly inject 100 μL of cell suspension (containing 0.5-1 x 106 cells), clamp gently the site of injection for a few seconds and slowly withdraw the needle without any sideways movement.
- Return the mouse back to its cage and monitor the recovery from anesthesia.
- Check tumor growth using a caliper for 3-4 weeks. Euthanize the animals when tumors reach approximately 0.5-1 cm3 using CO2 or according to locally-approved procedures.
4. Isolation of tumor interstitial fluid (TIF)
- Excision of subcutaneous tumors
- Block the limbs of the animals with paper tape and clean the skin with alcohol. Cut the skin open on the abdomen in order to separate it from the peritoneum and go on up to the limbs. Excise the tumor grown under the skin of the flank with the help of scissors, clamps and eventually a scalpel.
- Weigh the tumor and keep it in a clean tube on ice until following isolation of TIF.
- Isolation of TIF by centrifugation
- Cut the tumor in half, rinse the two parts quickly in PBS and blot them gently on filter paper to remove excess of PBS. Carry out these steps as fast as possible to avoid evaporation from the tumor.
- Immediately transfer the tumor into a 20 μm nylon cell strainer affixed atop a 50 mL conical tube.
- Centrifuge the tube at 400 x g for 10 min at 4 °C.
NOTE: This low speed centrifugation preserves cell integrity avoiding contamination of TIF by intracellular compartment. Intracellular content leakage can be tested in downstream applications for example by assessing the presence of intracellular housekeeping proteins, such as ribosomal proteins25. - Recover the TIF from the bottom of the tube, eventually aliquot it and immediately freeze it on dry ice and store at -80 °C until further analysis.
Optional: Based on the downstream proteomic analysis to be performed, dilute the sample in PBS with a protease inhibitor cocktail to avoid degradation of specific molecules.
NOTE: Depending on the composition of the tumor, in some cases very small tumors do not yield any fluid.
- Isolation of TIF by elution
- Cut the tumor into small pieces (≈1-3 mm3) with scissors or a scalpel and rinse carefully with cold PBS.
NOTE: In this step it is very important to work fast and perform minimum manipulation to avoid cell damage. - Transfer the tumor pieces into a 1.5 mL tube and add 500 μL of PBS with a protease inhibitor cocktail to avoid degradation of analytes. Incubate for 1 hour at 37 °C and 5% CO2.
- Recover the supernatant and transfer it to a new 1.5 mL tube. Centrifuge at 1,000 x g for 5 min at 4 °C to remove any cells from the sample.
- Transfer the supernatant to a new tube and centrifuge again at 2,000 x g for 8 min at 4 °C.
- Transfer the supernatant to a new tube and centrifuge again at 20,000 x g for 30 min at 4 °C to remove any debris. Recover the supernatant. Immediately aliquot and freeze TIF sample on dry ice and store at -80 °C until further analysis.
- Cut the tumor into small pieces (≈1-3 mm3) with scissors or a scalpel and rinse carefully with cold PBS.
Wyniki
We followed the procedure described above to obtain pancreatic juice from patients with PDAC (n=31) and other benign pancreatic afflictions (non-PDAC, n=9), including pancreatitis (n=2), papillary-ampulla tumors (n=4), neuroendocrine tumors (n=2), intraductal papillary mucinous neoplasia (IPMN; n=1)12. The pancreatic juice samples were then subjected to metabolomic analysis using nuclear magnetic resonance (1H-NMR)12. By filtering the broad NMR signa...
Dyskusje
In this study we have described the technique to intraoperatively collect pancreatic juice, a largely unexplored fluid biopsy. We have recently shown that pancreatic juice can be exploited as a source of metabolic markers of disease12. Metabolomic analysis on other liquid biopsies, such as blood5,6,7, urine8, and saliva9, have shown promising results in disc...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
We thank Roberta Migliore for technical assistance. The research leading to these results has received funding from Associazione Italiana per la ricerca sul cancro (AIRC) under IG2016-ID.18443 project – P.I. Marchesi Federica. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Materiały
| Name | Company | Catalog Number | Comments |
| 1 mL syringe | BD Biosciences | 309659 | |
| 1.5 mL Eppendorf tube | Greiner BioOne | GR616201 | |
| 20 µm nylon cell strainer | pluriSelect | 43-50020-03 | |
| 25G needle | BD Biosciences | 305122 | |
| 3 mL K2EDTA vacutainer | BD Biosciences | 366473 | |
| 3 mL syringe | BD Biosciences | 309656 | |
| 50 mL Falcon tube | Corning | 352098 | |
| Clamps | Medicon | 06.20.12 | |
| Disposable scalpel | Medicom | 9000-10 | |
| Fetal bovine serum | Microtech | MG10432 | |
| Flat-tipped forceps | Medicon | 06.00.10 | |
| Penicillin-Streptomycin | Lonza | ECB3001D | |
| Phosphate-Buffered Saline (PBS) | Sigma-Aldrich | D8537 | |
| Protease inhibitor cocktail | Roche | 34044100 | |
| RPMI medium | Euroclone | ECB9006L | |
| Scissors | Medicon | 02.04.09 | |
| Trypsin/EDTA 1x | Lonza | BE17-161F | |
| Ultraglutamine 100x | Lonza | BE17-605E/U1 |
Odniesienia
- Costello, E., Greenhalf, W., Neoptolemos, J. P. New biomarkers and targets in pancreatic cancer and their application to treatment. Nature Reviews Gastroenterology & Hepatology. 9 (8), 435-444 (2012).
- Siegel, R. L., Miller, K. D., Jemal, A. Cancer statistics, 2020. CA: A Cancer Journal for Clinicians. 70 (1), 7-30 (2020).
- Neoptolemos, J. P., et al. Therapeutic developments in pancreatic cancer: current and future perspectives. Nature Reviews Gastroenterology & Hepatology. 15 (6), 333-348 (2018).
- Sahin, I. H., Askan, G., Hu, Z. I., O'Reilly, E. M. Immunotherapy in pancreatic ductal adenocarcinoma: an emerging entity. Annals of Oncology. 28 (12), 2950-2961 (2017).
- Mayerle, J., et al. Metabolic biomarker signature to differentiate pancreatic ductal adenocarcinoma from chronic pancreatitis. Gut. 67 (1), 128-137 (2018).
- Bathe, O. F., et al. Feasibility of identifying pancreatic cancer based on serum metabolomics. Cancer Epidemiology, Biomarkers & Prevention. 20 (1), 140-147 (2011).
- Mayers, J. R., et al. Elevation of circulating branched-chain amino acids is an early event in human pancreatic adenocarcinoma development. Nature Medicine. 20 (10), 1193-1198 (2014).
- Napoli, C., et al. Urine metabolic signature of pancreatic ductal adenocarcinoma by (1)h nuclear magnetic resonance: identification, mapping, and evolution. Journal of Proteome Research. 11 (1), 1274-1283 (2012).
- Sugimoto, M., Wong, D. T., Hirayama, A., Soga, T., Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics. 6 (1), 78-95 (2010).
- Chen, R., et al. Comparison of pancreas juice proteins from cancer versus pancreatitis using quantitative proteomic analysis. Pancreas. 34 (1), 70-79 (2007).
- Mori, Y., et al. A minimally invasive and simple screening test for detection of pancreatic ductal adenocarcinoma using biomarkers in duodenal juice. Pancreas. 42 (2), 187-192 (2013).
- Cortese, N., et al. Metabolome of Pancreatic Juice Delineates Distinct Clinical Profiles of Pancreatic Cancer and Reveals a Link between Glucose Metabolism and PD-1+ Cells. Cancer Immunology Research. , (2020).
- Tanaka, M., et al. Cytologic Analysis of Pancreatic Juice Increases Specificity of Detection of Malignant IPMN-A Systematic Review. Clinical Gastroenterology and Hepatology. 17 (11), 2199-2211 (2019).
- Chen, K. T., et al. Potential prognostic biomarkers of pancreatic cancer. Pancreas. 43 (1), 22-27 (2014).
- Tian, M., et al. Proteomic analysis identifies MMP-9, DJ-1 and A1BG as overexpressed proteins in pancreatic juice from pancreatic ductal adenocarcinoma patients. BMC Cancer. 8, 241 (2008).
- Shi, C., et al. Sensitive and quantitative detection of KRAS2 gene mutations in pancreatic duct juice differentiates patients with pancreatic cancer from chronic pancreatitis, potential for early detection. Cancer Biology & Therapy. 7 (3), 353-360 (2008).
- Rogers, C. D., et al. Differentiating pancreatic lesions by microarray and QPCR analysis of pancreatic juice RNAs. Cancer Biology & Therapy. 5 (10), 1383-1389 (2006).
- Matsubayashi, H., et al. DNA methylation alterations in the pancreatic juice of patients with suspected pancreatic disease. Cancer Research. 66 (2), 1208-1217 (2006).
- Cote, G. A., et al. A pilot study to develop a diagnostic test for pancreatic ductal adenocarcinoma based on differential expression of select miRNA in plasma and bile. The American Journal of Gastroenterology. 109 (12), 1942-1952 (2014).
- Yu, J., et al. Digital next-generation sequencing identifies low-abundance mutations in pancreatic juice samples collected from the duodenum of patients with pancreatic cancer and intraductal papillary mucinous neoplasms. Gut. , (2016).
- Wiig, H., Swartz, M. A. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiological Reviews. 92 (3), 1005-1060 (2012).
- Haslene-Hox, H., et al. A new method for isolation of interstitial fluid from human solid tumors applied to proteomic analysis of ovarian carcinoma tissue. PLoS One. 6 (4), 19217 (2011).
- Zhang, J., et al. In-depth proteomic analysis of tissue interstitial fluid for hepatocellular carcinoma serum biomarker discovery. British Journal of Cancer. 117 (11), 1676-1684 (2017).
- Sullivan, M. R., et al. Quantification of microenvironmental metabolites in murine cancers reveals determinants of tumor nutrient availability. Elife. 8, (2019).
- Matas-Nadal, C., et al. Evaluation of Tumor Interstitial Fluid-Extraction Methods for Proteome Analysis: Comparison of Biopsy Elution versus Centrifugation. Journal of Proteome Research. 19 (7), 2598-2605 (2020).
- Espinoza, J. A., et al. Cytokine profiling of tumor interstitial fluid of the breast and its relationship with lymphocyte infiltration and clinicopathological characteristics. Oncoimmunology. 5 (12), 1248015 (2016).
- Halvorsen, A. R., et al. Profiling of microRNAs in tumor interstitial fluid of breast tumors - a novel resource to identify biomarkers for prognostic classification and detection of cancer. Molecular Oncology. 11 (2), 220-234 (2017).
- Yang, S., Huang, C. M. Recent advances in protein profiling of tissues and tissue fluids. Expert Review of Proteomics. 4, 515-529 (2007).
- Huang, C. M., et al. Mass spectrometric proteomics profiles of in vivo tumor secretomes: capillary ultrafiltration sampling of regressive tumor masses. Proteomics. 6 (22), 6107-6116 (2006).
- Leegsma-Vogt, G., Janle, E., Ash, S. R., Venema, K., Korf, J. Utilization of in vivo ultrafiltration in biomedical research and clinical applications. Life Sciences. 73 (16), 2005-2018 (2003).
- Schneiderheinze, J. M., Hogan, B. L. Selective in vivo and in vitro sampling of proteins using miniature ultrafiltration sampling probes. Analytical Chemistry. 68 (21), 3758-3762 (1996).
- Hardt, M., Lam, D. K., Dolan, J. C., Schmidt, B. L. Surveying proteolytic processes in human cancer microenvironments by microdialysis and activity-based mass spectrometry. Proteomics Clinical Applications. 5 (11-12), 636-643 (2011).
- Xu, B. J., et al. Microdialysis combined with proteomics for protein identification in breast tumor microenvironment in vivo. Cancer Microenvironment. 4 (1), 61-71 (2010).
- Bendrik, C., Dabrosin, C. Estradiol increases IL-8 secretion of normal human breast tissue and breast cancer in vivo. The Journal of Immunology. 182 (1), 371-378 (2009).
- Ao, X., Stenken, J. A. Microdialysis sampling of cytokines. Methods. 38 (4), 331-341 (2006).
- Ho, P. C., et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 162 (6), 1217-1228 (2015).
- Choi, J., et al. Intraperitoneal immunotherapy for metastatic ovarian carcinoma: Resistance of intratumoral collagen to antibody penetration. Clinical Cancer Research. 12 (6), 1906-1912 (2006).
- Wiig, H., Aukland, K., Tenstad, O. Isolation of interstitial fluid from rat mammary tumors by a centrifugation method. The American Journal of Physiology-Heart and Circulatory Physiology. 284 (1), 416-424 (2003).
- Li, S., Wang, R., Zhang, M., Wang, L., Cheng, S. Proteomic analysis of non-small cell lung cancer tissue interstitial fluids. World Journal of Surgical Oncology. 11, 173 (2013).
- Fijneman, R. J., et al. Proximal fluid proteome profiling of mouse colon tumors reveals biomarkers for early diagnosis of human colorectal cancer. Clinical Cancer Research. 18 (9), 2613-2624 (2012).
- Teng, P. N., Hood, B. L., Sun, M., Dhir, R., Conrads, T. P. Differential proteomic analysis of renal cell carcinoma tissue interstitial fluid. Journal of Proteome Research. 10 (3), 1333-1342 (2011).
- Turtoi, A., et al. Novel comprehensive approach for accessible biomarker identification and absolute quantification from precious human tissues. Journal of Proteome Research. 10 (7), 3160-3182 (2011).
- Wagner, M., Wiig, H. Tumor Interstitial Fluid Formation, Characterization, and Clinical Implications. Frontiers in Oncology. 5, 115 (2015).
- Haslene-Hox, H., Tenstad, O., Wiig, H. Interstitial fluid-a reflection of the tumor cell microenvironment and secretome. Biochimica Biophysica Acta. 1834 (11), 2336-2346 (2013).
- Hsieh, S. Y., et al. Secreted ERBB3 isoforms are serum markers for early hepatoma in patients with chronic hepatitis and cirrhosis. Journal of Proteome Research. 10, 4715-4724 (2011).
- Sun, W., et al. Characterization of the liver tissue interstitial fluid (TIF) proteome indicates potential for application in liver disease biomarker discovery. Journal of Proteome Research. 9 (2), 1020-1031 (2010).
- Haslene-Hox, H., et al. Increased WD-repeat containing protein 1 in interstitial fluid from ovarian carcinomas shown by comparative proteomic analysis of malignant and healthy gynecological tissue. Biochimica Biophysica Acta. 1834 (11), 2347-2359 (2013).
- Wang, T. H., et al. Stress-induced phosphoprotein 1 as a secreted biomarker for human ovarian cancer promotes cancer cell proliferation. Molecular & Cellular Proteomics. 9, 1873-1884 (2010).
- Gromov, P., et al. Up-regulated proteins in the fluid bathing the tumour cell microenvironment as potential serological markers for early detection of cancer of the breast. Molecular Oncology. 4 (1), 65-89 (2010).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone