Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Generation of a Simplified Three-Dimensional Skin-on-a-chip Model in a Micromachined Microfluidic Platform
W tym Artykule
Podsumowanie
Here, we present a protocol to generate a three-dimensional simplified and undifferentiated skin model using a micromachined microfluidic platform. A parallel flow approach allows the in situ deposition of a dermal compartment for the seeding of epithelial cells on top, all controlled by syringe pumps.
Streszczenie
This work presents a new, cost-effective, and reliable microfluidic platform with the potential to generate complex multilayered tissues. As a proof of concept, a simplified and undifferentiated human skin containing a dermal (stromal) and an epidermal (epithelial) compartment has been modelled. To accomplish this, a versatile and robust, vinyl-based device divided into two chambers has been developed, overcoming some of the drawbacks present in microfluidic devices based on polydimethylsiloxane (PDMS) for biomedical applications, such as the use of expensive and specialized equipment or the absorption of small, hydrophobic molecules and proteins. Moreover, a new method based on parallel flow was developed, enabling the in situ deposition of both the dermal and epidermal compartments. The skin construct consists of a fibrin matrix containing human primary fibroblasts and a monolayer of immortalized keratinocytes seeded on top, which is subsequently maintained under dynamic culture conditions. This new microfluidic platform opens the possibility to model human skin diseases and extrapolate the method to generate other complex tissues.
Wprowadzenie
Recently, advances have been made toward the development and production of in vitro human skin models for the analysis of the toxicity of cosmetic and pharmaceutical products1. Researchers in pharmaceutical and skin care industries have been using animals, mice being the most common, to test their products2,3,4,5. However, testing products on animals is not always predictive of the response in humans, which frequently leads to drug failure or adverse effects in humans and consequently to economic losses5,6. The UK was the first country that prohibited the use of animals for cosmetic testing in 1998. Later, in 2013, the EU banned the testing and approbation of cosmetics in animals (EU Cosmetics Regulation No. 1223/2009)7.
This prohibition is also being considered by other countries such as in 'The Humane Cosmetics Act' in the USA8. In addition to ethical concerns, the anatomical differences between animal and human skin make animal testing time-consuming, expensive, and often ineffective. Furthermore, the global in vitro toxicology testing market size is expected to reach USD 26.98 billion by 20259. For these reasons, there is a need to develop new methods and alternatives for those in vitro studies, such as bioengineered human skin models, that enable testing for safety and toxic effects of cosmetics and drugs without the use of animals.
There are two different kinds of commercially available, in vitro, human skin models. The first type consists of stratified epidermal equivalents containing multiple layers of differentiating keratinocytes that are seeded on different materials. Some of them have been approved by the Organization for Economic Co-operation and Development (OECD) and validated by the (European Centre for the Validation of Alternative Methods (ECVAM) for skin corrosion and irritation testing, such as EpiDerm or SkinEthic10,11,12. The second type are full-skin equivalents with a layer of differentiating human keratinocytes seeded on a three-dimensional (3D) scaffold that contains fibroblasts, such as T-Skin and EpiDerm-FT. However, these models are cultured under static conditions, which makes them unable to accurately represent human physiological conditions.
Recent interest has focused on generating in vitro 3D skin models in cell culture-insert (CCI) formats with dynamic perfusion13,14,15,16,17,18,19. However, these systems cannot be considered stricto sensu as microfluidic skin-on-chips as per their classical definition in the field. Ingber's definition for organs-on-a-chip states that the organ must be placed inside the microfluidic channels, which is a condition that only a few devices fulfil20,21. Skin-on-chips have so far modelled mostly simple epithelia as single-cell layers and/or dermal cell layers separated by a porous membrane22,23. Although there have been some advances modeling skin in microfluidic systems16,24, there is currently no literature showing an organ-on-a-chip system that fits Ingber's definition, capable of producing a multilayered skin in situ and including both epithelial and stromal components.
In this work, a new, cost-effective, robust, vinyl-based microfluidic platform for skin-on-a-chip applications is presented. This platform was produced by micro-machining, which provides more simplicity in the fabrication process, as well as increased flexibility and versatility in the layout of the device, overcoming some of the limitations of PDMS25. A way to introduce a simplified skin construct through a parallel flow controlled with syringe pumps was also designed. Parallel flow allows two fluids with very different viscosities (a buffer and fibrin pre-gel in this case) to be perfused through a channel without mixing with each other. As a proof of concept, a dermo-epidermal construct containing fibroblasts embedded in a fibrin matrix mimicking the dermis was introduced in the device, on top of which a monolayer of keratinocytes was loaded to emulate the undifferentiated epidermis. The dermal compartment height can be modulated by modifying the flow rates. The main novelty of this work, compared to previously described models22,26,27,28,29, is the development of a 3D construct inside a microchamber by means of microfluidics. Although this article presents a simplified undifferentiated skin, the long-term goal is to generate and characterize a fully differentiated skin construct to demonstrate its viability and functionality for drug and cosmetic testing purposes.
Protokół
1. Chip design and micromachining parameters
- Design the microfluidic chip layers with FreeCAD open-source design software; refer to Table 1 for the dimensions of the channels. Include four 2.54 mm diameter holes in the design to use a custom-made aligner for a correct layer superposition.
| Length (μm) | Width (μm) | |
| Lower chamber | 28,400 | 800 |
| Upper chamber | 31,000 | 800 |
Table 1: Dimensions of the upper and lower channels of the device.
- Cut 95 µm-thick, adhesive, transparent vinyl sheets into 30 cm x 30 cm squares to fit in the plotter properly.
- Use Brother CanvasWorkspace software to create a 30 cm x 30 cm workspace, and fill it with the designed patterns for the different layers of the chip (Figure 1A). Store it in a .svg file.
- Cut the 30 cm x 30 cm vinyl sheets with the edge plotter (Figure 1B-D).
- Stick the vinyl sheet to a low tack adhesive mat, and eliminate all the air bubbles if necessary.
- Upload the .svg file to the plotter, and set the cutting parameters: cutting blade: level 3; cutting pressure: level 0; cutting speed: level 1. Place the adhesive mat with the vinyl into the plotter, and start the cutting process.
- Cut the top channel pattern on 12 µm-thick double-sided tape vinyl by following the previous steps.
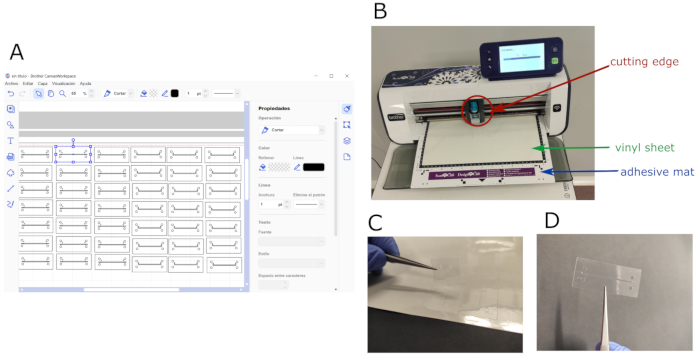
Figure 1: Chip design and micromachining process. (A) Software layout showing the working space filled with both the top and bottom patterns designed for the chip. (B) Edge plotter during cutting process; cutting blade, whole vinyl sheet, and adhesive mat are shown. (C) Patterned vinyl being detached from the cut sheet. (D) Sample of an adhesive vinyl layer patterned with the top channel design. Please click here to view a larger version of this figure.
2. PDMS layer fabrication
- Mix the PDMS and curing agent in a ratio of 10:1 (v/v), and place the mixture under vacuum for 15 min to remove air bubbles. Pour 55 mL of the mixture into a 55 cm2 square culture dish to obtain a 2 mm-thick layer. Remove bubbles with a needle.
- Cure the mixture (step 2.1) in an oven for 1 h at 80 °C. Unmold the PDMS and cut it into rectangles with the chip dimensions. Make holes for the tubing with an 18 G syringe needle.
3. Chip assembly
NOTE: For better understanding, see Figure 2.
- Assemble the whole device using an aligner to adjust channels, inlets, and outlets properly. Pile up four vinyl layers (with the corresponding bottom micropattern) for assembling the lower channel, keeping the cover tape of the bottom layer to avoid sticking to the aligner.
- Cut and place the polycarbonate (PC) porous membrane on top of the lower channel to separate it from the upper one. Be careful not to cover the inlets of the lower channel.
- Add ten vinyl layers with the upper chamber design. Stick a double-sided tape vinyl layer with the top-channel pattern on top. Remove the chip from the aligner and stick it on the glass slide.
- Place a 2-mm-thick PDMS sheet on top of the double-sided tape vinyl layer to provide appropriate anchoring for the tubing and to avoid leakage. Leave a weight on top of the chip overnight to ensure the chip is completely watertight. Sterilize the chip by flushing 70% v/v ethanol for 5 min, and then wash with distilled H2O.
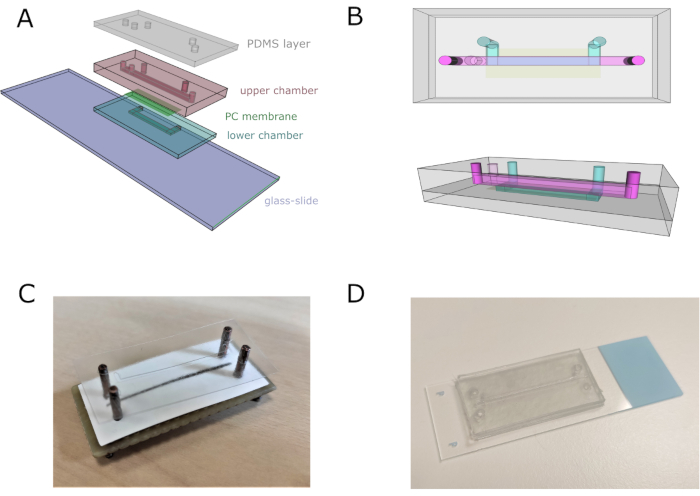
Figure 2: Microfluidic chip assembly. (A) General scheme of the assembly of the device. Lower and upper chambers are composed of four and eleven superimposed vinyl sheets, respectively. (B) Top and lateral views of the microfluidic chip. Top and bottom channels are represented in pink and blue, respectively. (C) Image of the chip assembly using a custom-made aligner. (D) Chip image after complete assembly. Please click here to view a larger version of this figure.
4. Pump connections
NOTE: The graphical representation of pumps connections is shown in Figure 3.
- Connect Pump 1 to the Upper Chamber Inlet 1 (UCi1).
- Connect Pump 2 to the Upper Chamber Inlet 2 (UCi2).
- Connect Pump 3 to the Lower Chamber Inlet (LCi).
- Connect Upper Chamber Outlet (UCo) and Lower Chamber Outlet (LCo) to a waste tube.
- Connect the syringes to each inlet using polytetrafluoroethylene (PTFE) tubes and 18 G stainless steel connectors.
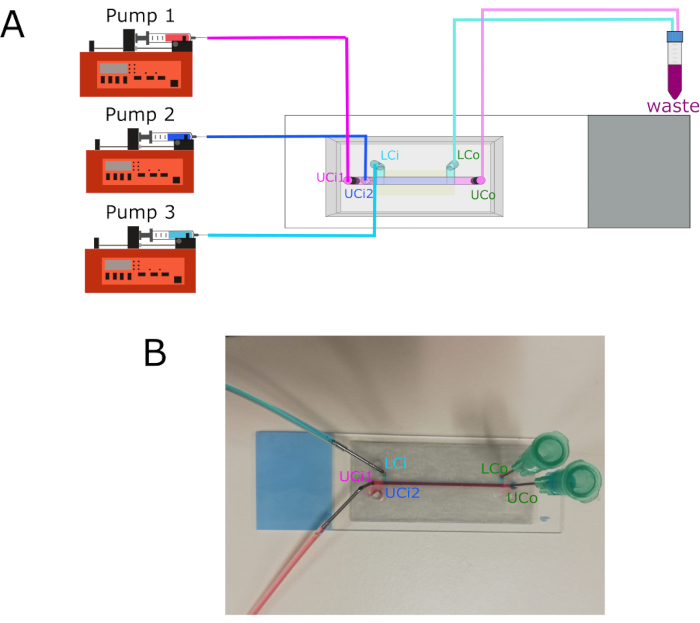
Figure 3: Pump connections and inlets/outlets location. (A) Diagram showing the connection of the three different pumps to their respective inlets. Outlets connect to a waste container. (B) Chip image with labeled inlets and outlets. Abbreviations: LCi = lower chamber inlet; LCo = lower chamber outlet; UCi1 = upper chamber inlet 1; UCi2 = upper chamber inlet 2; UCo = upper chamber outlet. Please click here to view a larger version of this figure.
5. Cell culture
NOTE: The HaCaT cell line has a commercial origin. Human primary fibroblasts come from healthy donors and were obtained from the collection of biological samples of human origin registered in "Registro Nacional de Biobancos para Investigación Biomédica del Instituto de Salud Carlos III".
- Work in a cell culture hood, previously sterilized under ultraviolet light and wiped with ethanol.
- Thaw H2B-GFP-HaCaT cells (human immortalized skin keratinocytes, hKCs) and GFP-human primary fibroblasts (hFBs) at 37 °C, add 2 mL of culture medium, and centrifuge for 7 min at 20 °C at 250 × g.
NOTE: H2B-GFP-HaCaT cells are human immortalized keratinocytes modified to express a hybrid histone H2B-green fluorescent protein (GFP), providing their nuclei with green fluorescence. GFP-hFBs are human primary fibroblasts transformed with the vector pLZRS-IRES-EGFP to express cytoplasmic green fluorescence. These cells were modified following previously published protocols30,31 - Culture both hKCs and hFBs in 1x DMEM supplemented with 10% fetal bovine serum and 1% of antibiotic/antimycotic solution. Pre-warm the culture medium at 37 °C before use.
- Detach the cells by washing them with 1x phosphate-buffered saline (PBS), adding 2 mL of trypsin/ethylenediamine tetraacetic acid (EDTA) and incubating them for 10 min at 37 °C.
- Inactivate trypsin adding 4 mL of culture medium. Resuspend the cells, and transfer them to a 15 mL tube. Remove 10 µL to count cells on a Neubauer chamber and determine the appropriate concentration.
- Centrifuge the 15 mL tube for 7 min at 20 °C at 250 × g. Remove the supernatant, and resuspend the pellets at the desired concentration: hFBs at 50,000 cells/mL and hKCs at 5·× 106 cells/mL.
6. Fibrinogen pre-gel preparation
- Activate thrombin by adding 1mL of CaCl2 (1% w/v in NaCl) to the vial.
- Add the following components to obtain 1 mL of a fibrin hydrogel at a final concentration of fibrin of 3.5 mg/mL: 59 µL of activated thrombin (10 NIH units/mL), 59 µL of tranexamic acid (Table of Materials, 100 mg/mL), 764 µL of culture medium containing 50,000 hFBs/mL, 118 µL of fibrinogen (20 mg/mL in NaCl (0.9% w/v)).
NOTE: Fibrinogen must be added at the last moment.
7. Parallel flow protocol
- Pump 1x PBS with pump 3 through the LCi at 50 µL/min during the whole process.
- Pump sacrificial fluid (1x PBS) with pump 2 through the UCi2 at 100 µL/min.
- Load the syringe with the pre-gel, rapidly place it into pump 1, and run it at 200 µL/min (Figure 4A).
- Stop pumps 1 and 2 once the pre-gel exits the UCo.
- Leave the chip without removing the tubing at 37 °C for at least 10 min to allow gelation.
- Pump culture medium at 50 µL/h with pump 3 through UCi2 overnight.
- Block UCi1 with a cap.
8. hKCs monolayer seeding
- Check under the microscope that hFBs are spread 24 h after the generation of the dermal compartment.
- Introduce the hKCs with pump 2 through UCi2 at 5 ×·106 cells/mL at 40 µL/min for 1 min (Figure 4B).
- Leave the chip overnight at 37 °C in a humidity-saturated incubator for cell attachment.
- Pump fresh culture medium with pump 3 only through LCi at 50 µL/min (Figure 4C).
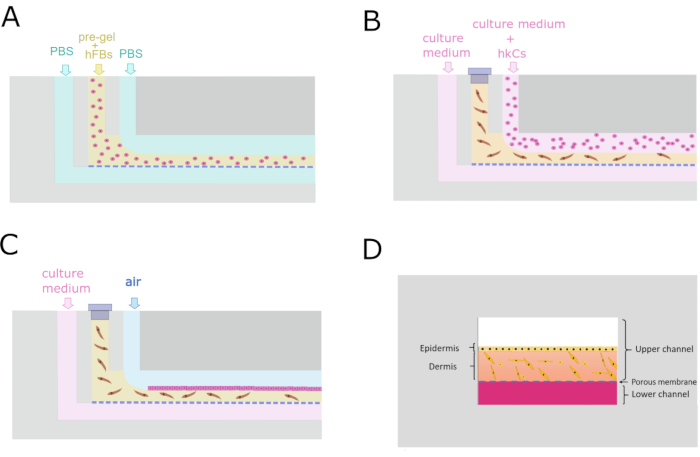
Figure 4: Microfluidic protocol for the generation of the dermo-epidermal construct. (A) Transverse cross-section showing the parallel flow process to generate the dermal compartment. (B) Keratinocyte monolayer seeding 24 h after dermal compartment generation. (C) Cell culture maintenance inside the microfluidic device. (D) Cross-sectional recreation of the skin inside the chip. Please click here to view a larger version of this figure.
9. Cell viability assay
NOTE: Live/Dead kit stains cells with green or red fluorescence depending on their live or dead state. For proper viability differentiation, non-fluorescent hKCs and hFBs must be used in this step. All the steps in the procedure are carried out through UCi2 with pump 2.
- Wash the top channel with 1x PBS for 5 min at 50 µL/min to remove culture medium.
- Pump air to remove the 1x PBS at 50 µL/min.
- Prepare Calcein AM/Ethidium homodimer-1 Kit (Live/Dead) solution by following the manufacturer's instructions.
- Pump Live/Dead solution at 50 µL/min for 2 min.
- Incubate 30 min at 37 °C in the dark.
- Wash top channel by pumping 1x PBS at 50 µL/min for 2 min to remove any remaining reagent.
- Observe the sample under the confocal microscope. Use an excitation wavelength of 495/590 nm and an emission wavelength of 519/617 nm for live and dead cells, respectively.
Wyniki
The designed chip is composed of two fluidic chambers separated by a 5 µm pore size PC membrane that allows the growth of the cell by allowing the passage of growth-promoting molecules from the lower chamber. The upper chamber holds the tissue construct, in this case, a monolayer of hKCs on a fibrin hydrogel containing hFBs.
The height of the channels is determined by the number of adhesive sheets added to each channel. The lower chamber is composed of 4 layers (380 µm) and the upper...
Dyskusje
The motivation to develop this method was the desire to model skin diseases and study the effects of new and innovative therapies in a high-throughput platform. To date, this laboratory produces these dermo-epidermal equivalents by casting-either manually or with the help of the 3D bioprinting technology-the fibrin gel with fibroblasts into a cell culture insert plate and seeding the keratinocytes on top of it. Once the keratinocytes reach confluence, the 3D culture is exposed to the air-liquid interface, which induces k...
Ujawnienia
The authors declare that they have no competing financial interests.
Podziękowania
We sincerely thank Dr. Javier Rodríguez, Dr. María Luisa López, Carlos Matellán, and Juan Francisco Rodríguez for very helpful suggestions, discussions, and/or preliminary data. We also kindly thank the contributions of Sergio Férnandez, Pedro Herreros, and Lara Stolzenburg to this project. Special thanks go to Dr. Marta García for GFP-labelled hFBs and hKCs. Finally, we recognize the excellent technical assistance of Guillermo Vizcaíno and Angélica Corral. This work was supported by the "Programa de Actividades de I+D entre Grupos de Investigación de la Comunidad de Madrid", Project S2018/BAA-4480, Biopieltec-CM. This work was also supported by the "Programa de excelencia", Project EPUC3M03, CAM. CONSEJERÍA DE EDUCACIÓN E INVESTIGACIÓN.
Materiały
| Name | Company | Catalog Number | Comments |
| Amchafibrin | Rottafarm | Tranexamic acid | |
| Antibiotic/antimycotic | Thermo Scientific HyClone | ||
| Calcium chloride | Sigma Aldrich | ||
| Culture plates | Fisher | ||
| DMEM | Invitrogen Life Technologies | ||
| Double-sided tape vynil | ATP Adhesive Systems | GM 107CC, 12 µm thick | |
| Edge plotter | Brother | Scanncut CM900 | |
| FBS | Thermo Scientific HyClone | ||
| Fibrinogen | Sigma Aldrich | Extracted from human plasma | |
| Glass slide | Thermo Scientific | ||
| GFP-Human dermal fibroblasts | - | Primary. Gift from Dr. Marta García | |
| H2B-GFP-HaCaT cell line | ATCC | Immortalized keratinocytes. Gift from Dr. Marta García | |
| Live/dead kit | Invitrogen | ||
| PBS | Sigma Aldrich | ||
| Polycarbonate membrane | Merk TM | 5 µm pore size | |
| Polydimethylsiloxane | Dow Corning | Sylgard 184 | |
| Sodium chloride | Sigma Aldrich | ||
| Syringes | Terumo | 5 mL | |
| Thrombin | Sigma Aldrich | 10 NIH/vial | |
| Transparent adhesive vinyl | Mactac | JT 8500 CG-RT, 95 µm thick | |
| Trypsin/EDTA | Sigma Aldrich | ||
| Tubing | IDEX | Teflon, 1/16” OD, 0.020” ID |
Odniesienia
- McNamee, P., et al. A tiered approach to the use of alternatives to animal testing for the safety assessment of cosmetics: Eye irritation. Regulatory Toxicology and Pharmacology. 54 (2), 197-209 (2009).
- Mathes, S. H., Ruffner, H., Graf-Hausner, U. The use of skin models in drug development. Advanced Drug Delivery Reviews. 69-70, 81-102 (2014).
- Abd, E., et al. Skin models for the testing of transdermal drugs. Clinical Pharmacology: Advances and Applications. 8, 163-176 (2016).
- Flaten, G. E., et al. In vitro skin models as a tool in optimization of drug formulation. European Journal of Pharmaceutical Sciences. 75, 10-24 (2015).
- Avci, P., et al. Animal models of skin disease for drug discovery. Expert Opinion on Drug Discovery. 8 (3), 331-355 (2014).
- Mak, I. W., Evaniew, N., Ghert, M. Lost in translation: animal models and clinical trials in cancer treatment. American Journal of Translational Research. 6 (2), 114-118 (2014).
- Pronko, P. P., VanRompay, P. A., Zhang, Z., Nees, J. A. Pronko et al. Reply. Physical Review Letters. 86 (7-12), 1387 (2001).
- H.R.2858 - Humane Cosmetics Act. 114th Congress Available from: https://congress.gov/bill/114th-congress/house-bill/2858 (2016)
- . Global in-vitro toxicology testing market report: size, share & trends analysis 2014-2015 Available from: https://www.prnewswire.com/news-releases/global-in-vitro-toxicology-testing-market-report-size-share--trends-analysis-2014-2025-300704958.html (2018)
- Zhang, Z., Michniak-Kohn, B. B. Tissue engineered human skin equivalents. Pharmaceutics. 4 (1), 26-41 (2012).
- OECD. In vitro skin corrosion: reconstructed human epidermis (RhE) test method. Test Guideline No.431. OECD Guideline for Testing of Chemicals. , (2019).
- Almeida, A., Sarmento, B., Rodrigues, F. Insights on in vitro models for safety and toxicity assessment of cosmetic ingredients. International Journal of Pharmaceutics. 519 (1-2), 178-185 (2017).
- vanden Broek, L. J., Bergers, L. I. J. C., Reijnders, C. M. A., Gibbs, S. Progress and future Prospectives in Skin-on-Chip Development with Emphasis on the use of Different Cell Types and Technical Challenges. Stem Cell Reviews and Reports. 13 (3), 418-429 (2017).
- Ataç, B., et al. Skin and hair on-a-chip: In vitro skin models versus ex vivo tissue maintenance with dynamic perfusion. Lab on a Chip. 13 (18), 3555-3561 (2013).
- Abaci, H. E., Gledhill, K., Guo, Z., Christiano, A. M., Shuler, M. L. Pumpless microfluidic platform for drug testing on human skin equivalents. Lab on a Chip. 15 (3), 882-888 (2015).
- Wu, R., et al. Full-thickness human skin-on-chip with enhanced epidermal morphogenesis and barrier function. Materials Today. 21 (4), 326-340 (2017).
- Materne, E. -. M., et al. The multi-organ chip - a microfluidic platform for long-term multi-tissue coculture. Journal of Visualized Experiments: JoVE. (98), e52526 (2015).
- Schimek, K., et al. Bioengineering of a full-thickness skin equivalent in a 96-well insert format for substance permeation studies and organ-on-a-chip applications. Bioengineering. 5 (2), 43 (2018).
- Alberti, M., et al. Multi-chamber microfluidic platform for high-precision skin permeation testing. Lab on a Chip. 17, 1625-1634 (2017).
- Bhatia, S. N., Ingber, D. E. Microfluidic organs-on-chips. Nature BIotechnology. 32 (8), 760-772 (2014).
- Huh, D., Hamilton, G. A., Ingber, D. E. From 3D cell culture to organs-on-chips. Trends in Cell Biology. 21 (12), 745-754 (2011).
- Wufuer, M., et al. Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Scientific Reports. 6, 37471 (2016).
- Ramadana, Q., Ting, F. C. W. In vitro micro-physiological immune-competent model of the human skin. Lab on a Chip. 16, 1899-1908 (2016).
- Kim, K., Jeon, H. M., Choi, K. C., Sung, G. Y. Testing the effectiveness of Curcuma longa leaf extract on a skin equivalent using a pumpless skin-on-a-chip model. International Journal of Molecular Sciences. 21 (11), 3898 (2020).
- Halldorsson, S., Lucumi, E., Gómez-Sjöberg, R., Fleming, R. M. T. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosensors and Bioelectronics. 63, 218-231 (2015).
- Huh, D., Matthews, B. D., Mammoto, A., Montoya-Zavala, M., Hsin, H. Y. Reconstituting organ-level lung functions on a chip. Science. 328 (5986), 1662-1668 (2010).
- Huh, D. A human disease model of drug toxicity - induced pulmonary edema in a lung-on-a-chip microdevice. Scientific Translational Medicine. 4 (159), (2012).
- Beckwitt, C. H., et al. Liver ' organ on a chip '. Experimental Cell Research. 363 (1), 15-25 (2018).
- Poceviciute, R., Ismagilov, R. F. Human-gut-microbiome on a chip. Nature Biomedical Engineering. 3 (7), 500-501 (2019).
- Kanda, T., Sullivan, K. F., Wahl, G. M. Histone-GFP fusion protein enables sensitive analysis of chromosome dynamics in living mammalian cells. Current Biology. 8 (7), 377-385 (1998).
- Escámez, M. J., et al. Assessment of optimal virus-mediated growth factor gene delivery for human cutaneous wound healing enhancement. Journal of Investigative Dermatology. 128 (6), 1565-1575 (2008).
- Llames, S. G., et al. Human plasma as a dermal scaffold for the generation of a completely autologous bioengineered skin. Transplantation. 77 (3), 350-355 (2004).
- Llames, S., et al. Clinical results of an autologous engineered skin. Cell Tissue Bank. 7 (1), 47-53 (2006).
- Cubo, N., Garcia, M., del Cañizo, J. F., Velasco, D., Jorcano, J. L. 3D bioprinting of functional human skin: production and in vivo analysis. Biofabrication. 9 (1), 015006 (2016).
- Mori, N., Morimoto, Y., Takeuchi, S. Skin integrated with perfusable vascular channels on a chip. Biomaterials. 116, 48-56 (2017).
- Kim, H. J., Li, H., Collins, J. J., Ingber, D. E. Contributions of microbiome and mechanical deformation to intestinal bacterial overgrowth and inflammation in a human gut-on-a-chip. Proceedings of the National Academy of Sciences of the United States of America. 113 (1), 7-15 (2016).
- Shah, P., et al. A microfluidics-based in vitro model of the gastrointestinal human-microbe interface. Nature Communications. 7, 11535 (2016).
- Marx, U., et al. Human-on-a-chip' developments: A translational cuttingedge alternative to systemic safety assessment and efficiency evaluation of substances in laboratory animals and man. Alternatives to Laboratory Animals. 40 (5), 235-257 (2012).
- Bein, A., et al. Microfluidic organ-on-a-chip models of human intestine. Cellular and Molecular Gastroenterology and Hepatology. 5 (4), 659-668 (2018).
- Bennet, D., Estlack, Z., Reid, T., Kim, J. A microengineered human corneal epithelium-on-a-chip for eye drops mass transport evaluation. Lab on a Chip. 18, 1539-1551 (2018).
- Kim, H. J., Huh, D., Hamilton, G., Ingber, D. E. Human gut-on-a-chip inhabited by microbial flora that experiences intestinal peristalsis-like motions and flow. Lab on a chip. 12, 2165-2174 (2012).
- Kim, H. J., Ingber, D. E. Gut-on-a-chip microenvironment induces human intestinal cells to undergo villus differentiation. Integrative Biology. 5 (9), 1130-1140 (2013).
- O'Neill, A. T., Monteiro-Riviere, N. A., Walker, G. M. Characterization of microfluidic human epidermal keratinocyte culture. Cytotechnology. 56 (3), 197-207 (2008).
- Ren, K., Chen, Y., Wu, H. New materials for microfluidics in biology. Current Opinion in Biotechnology. 25, 78-85 (2014).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone