Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Development of a Lateral Flow Immunochromatographic Strip for Rapid and Quantitative Detection of Small Molecule Compounds
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Streszczenie
Membrane-based lateral flow immunochromatographic strips (ICSs) are useful tools for low-cost self-diagnosis and have been efficiently applied to toxin, physiological index and clinical biomarker detection. In this protocol, we provide a detailed description of the steps to develop a rapid, sensitive and quantitative lateral-flow immunoassay (using AuNPs as a marker and mAbs as a probe). The procedure describes the preparation and characterization of colloidal gold, synthesis of the AuNP-mAb conjugate, assembly of the immunochromatographic strip, and methodological investigation of the assay. The results showed that the final strips can be further utilized for the rapid and convenient self-diagnosis of a small molecule, which may provide an alternative tool in the rapid and precise analysis of physiological and biological indices.
Wprowadzenie
Membrane-based lateral flow immunochromatographic strips (ICSs) are useful tools for low-cost and rapid detection. The nitrocellulose membrane as the carrier and colloidal gold as markers of immune chromatography rapid diagnostic reagents are the most commonly used POCT (point of care testing) method, and the testing scope of the project is wider. From their original application in monitoring during pregnancy, their use has been extended to monitor blood coagulation state1,2, myocardial injury3, veterinary medicine4, pesticide residues5, infectious diseases6 and drug concentrations. More types of samples can be assessed, including urine, saliva, whole blood, serum and other body fluids7,8,9.
In recent years, numerous novel assays have been developed for detecting biomarkers in the diagnosis of disorders, including HPLC, UPLC, LC-MS and ELISA, which are sensitive and accurate, credible and specific. However, these methods require sophisticated instrumentation, complex preprocessing and time-consuming treatments9. Hence, developing a more rapid and convenient point-of-care diagnostic strategy for the self- and real-time detection of medicinal active compounds is urgent10,11.
The popularity of ICSs, especially for common tests, is driven by their ease of use, as they do not require professionals or elaborate instrumental setups12. In other words, people who do not have special training can operate strips or self-tests13. The results of the test can be obtained in 5 minutes, which means it can be used for site inspections14. Moreover, according to our calculations, the cost of strips could be lower than 1 RMB15, which means that the tests are inexpensive to promote16. Hence, the ICS is a relatively accurate, simple, and inexpensive disposable device. ICSs based on colloidal gold17,18 are also applied in rapid COVID-19 detection.
The principle of ICS can be divided into sandwich ICS and competitive ICS. Figure 1A is a schematic diagram of the sandwich ICS, which is mainly used for detecting macromolecular substances such as proteins, including tumor markers, inflammatory factors, and human chorionic gonadotropin (HCG, early pregnancy antigen). In this method, paired antibodies targeted at different epitopes of the antigen are used, and the capture antibody is dried on the NC membrane as a test line. Labeled antibody is dried on the conjugate pad, and secondary antibody is used as the control line.
Figure 1B is a schematic diagram of competitive ICS, which is mainly used to detect small molecule substances (MWCO < 2000 Da). The coating antigen is fixed on the NC membrane as a test line, and the labeled antibody is dried on the conjugate pad. During detection, the sample and labeled antibody flow through the detection line under capillary action, and the coated antigen competitively binds free antigen in the sample and develops a red color on the detection line.
Recently, we described the procedure of monoclonal antibody generation against natural products19. In this work, we developed a novel lateral flow immunoassay based on the prepared anti-SSD mA20 for rapid, on-site detection. The results indicate that the immunochromatography assay is an indispensable and convenient tool for detecting natural product-derived compounds.
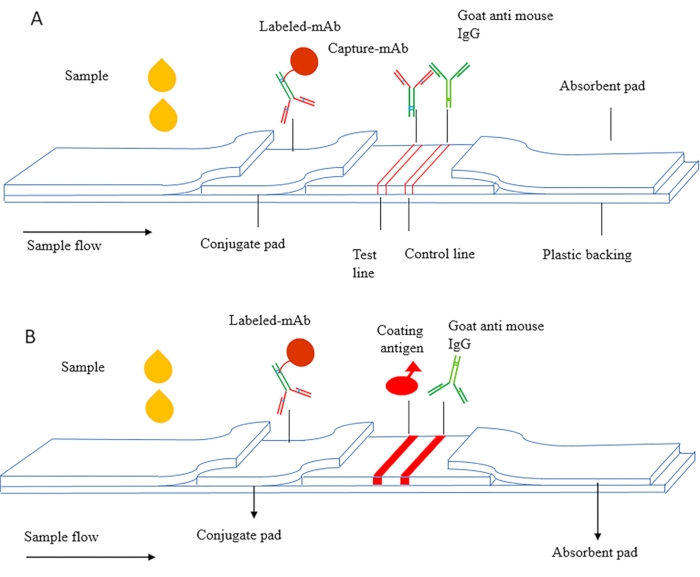
Figure 1 Schematic diagram of the immunochromatography assay (A) Sandwich immunochromatographic test strips. (B) Indirect competitive immune chromatographic test strips. This figure has been modified from Zhang et al., 201821. Please click here to view a larger version of this figure.
Protokół
All of the procedures performed in this study were approved by the Ethics Review Committee at the Beijing University of Chinese Medicine (approval number 2017BZYYL00120).
1. Preparation and Characterization of Colloidal Gold
NOTE: For colloidal gold synthesis, as colloidal gold is easily adsorbed on the inner wall of the vessel and is prone to precipitation by impurities, the vessel for synthesis and storage of colloidal gold should be thoroughly cleaned and soaked in acid (40 mL of distilled water, 360 mL of concentrated sulfuric acid, 20 g of potassium dichromate) or subjected to surface passivation treatment. A citric acid reduction method was used to synthesize colloidal gold.
- Turn on the magnetic stirrer and place the flask (250 mL) on the mixer.
- Prepare 4% gold chloride acid solution and 1% sodium citrate solution, respectively.
- Add 120 mL of distilled water to a round bottom flask and heat it to boiling on a thermostatic magnetic stirrer.
- Keep boiling, and quickly add 0.5 mL of 4% chloroauric acid and 5 mL of 1% sodium citrate.
- Observe the color of the solution. The pale yellow chloroauric acid solution turns wine red within a few minutes.
- Continue heating for 10 minutes, until the solution changes from colorless to transparent wine red.
- Turn off the power of the thermostatic magnetic mixer, cool to room temperature, and move the mixture to a clean bottle. Store it at 4 °C.
- Determine the size and morphology of the AuNPs by ultraviolet spectroscopy and TEM imaging.
NOTE: Different sizes of colloid gold particles for various applications can be obtained by changing the proportion of citrate sodium and chloroauric acid.
2. Synthesis of AuNPs-mAb Conjugate
NOTE: Since antibodies bind to colloidal gold by electrostatic adsorption, charges on the surface of proteins and colloidal gold directly affect the binding intensity; therefore, the buffer pH value is an important factor affecting the stability of the antibody-colloidal gold conjugate. SSD and anti-SSD mAbs are used as examples in this protocol.
- Evaluation of coupling pH
- Add 100 μL of NaCl solution (10% m/v) into eight tubes.
- Adjust the AuNP solutions at pH 5, 6, 7, 8, 9, 10, 11 and 12 with 0.1 M K2CO3.
- Add 100 μL of colloidal gold solution (pH adjusted 5-12) to eight tubes containing NaCl.
- Allow the solutions to stand for several minutes after blending. Observe the color change of each tube solution, and record the tube that remains red.
- Choose the pH value with the least addition of K2CO3 and stable solution color as the optimum pH value for preparing AuNP-mAb conjugates.
NOTE: Do not use a pH meter because the probe may be destroyed by AuNPs.
- Evaluation of antibody amount
- Add 100 μL of NaCl solution (10% m/v) to eight tubes.
- Add 100 μL of colloidal gold solution with optimum pH to each tube.
- Add the monoclonal antibody solutions (protein concentration 0.1 mg/mL-3.2 mg/mL) to the abovementioned eight tubes.
- Allow the solutions to stand for several minutes after blending. Observe the color change of each tube solution, and record the tube that remains red.
- Choose the antibody amount with the lowest concentration of mAb and stable solution color as the optimum mAb amount for preparing AuNP-mAb conjugates.
- Prepare the resuspension buffer: add 1 M Tris-HCl (pH 8.8), 1% (w/v) BSA, 0.5% (v/v) Tween 20 and 1% (v/v) PEG 20000 and blend well.
- Synthesis of AuNP-mAb conjugate
- Take 10 mL of colloidal gold solution and use 0.1 M K2CO3 to adjust the solution to the optimal pH value.
- Slowly add SSD mAbs at appropriate concentrations and shake at room temperature for 30 min.
- Centrifuge the mixture at 83 x g (1,000 rpm) for 10 min at 4 °C. Remove the precipitate, which contains impurities or precipitated colloidal gold.
- Centrifuge the mixture at 8,330 x g (10,000 rpm) for 30 min at 4 °C. Discard the supernatant, and the precipitate is the colloidal gold-mAb conjugate.
- Add the resuspension buffer to dissociate the precipitation.
3. Assembly of the Strip
NOTE: For later flow immunoassays, the selection and pretreatment of membrane material will directly affect the test, which should be investigated. The immunochromatographic strip consists of a sample pad, a conjugate pad, a nitrocellulose (NC) membrane, an absorbent pad and a PVC board (Figure 1). The membrane material should be checked and evaluated by stereomicroscopy to eliminate inhomogeneity.
- Paste the NC membrane on a PVC board 2 cm apart from the edge of the suction end of the board.
- Add SSD-BSA (2 mg/mL) dropwise to the NC membrane (2 cm apart from the upper edge) as a test line (1 mm wide), and add goat anti-mouse IgG (1.5 mg/mL) dropwise on the NC membrane (2 cm apart from the lower edge) as a control line (1 mm wide). Control the amount of protein added.
- Attach the absorbent pad to the PVC sheet above the NC membrane and overlap it with the NC membrane by 2 mm.
- Submerge the glass fiber membrane into the AuNPs-mAb conjugate solution. Dry the wet membrane in an incubator at 37 °C.
- Trim the glass fiber membrane to 5 cm long and 2 cm wide and use it as a conjugate pad.
- Paste the pretreated conjugate pad under the NC membrane. The length of overlap with the NC membrane should be 0.1 cm.
NOTE: The glass fiber membrane has a strong ability to bind and release proteins. - Trim the fusion 3 membrane to 1.8 cm long and 3.5 cm wide and use it as the sample pad.
- Paste the sample pad on the PVC board and overlap it with the conjugate pad by 2 mm.
- Cut the assembled paper board into 3.5-mm-wide strips using a cutting machine and compact it using a batch lamination system.
- Finally, place the test strips into the shell, seal them in an aluminum foil bag containing desiccant, and store them away from light. The ICSs are now assembled.
NOTE: The above is the laboratory procedure. In production, gold spraying equipment and cross-membrane instruments are used to spray gold and make the T and C lines.
4. Quantitative Detection
- Drop 50 µL of sample solution onto the sample hole to observe the chromatography process.
NOTE: As a result of the capillary action driven by the absorbent pad, the sample solution migrates to the other end of the strip. When the sample solution reaches the conjugate pad, the SSD (antigen) in the sample reacts with the AuNPs-mAb preloaded on the pad. When the solution migrates and reaches the T-line, the AuNPs-mAb without SSD can be selectively captured by SSD-BSA (antigen-carrier protein conjugate), showing as a red color on the T-line. Then, the solution migrates to the C line, where the AuNPs-mAb are captured by goat anti-mouse IgG in the region, thus showing as a red color on the C line. - Analyze the strips with a portable strip reader. The machine can provide the ratio of the test line to the control line (T/C).
- Evaluate the specificity, sensitivity, repeatability and stability of the ICS test.
NOTE: Under qualitative detection, one red line indicates a positive result (control line). Two red lines indicate a negative result (test and control lines). If the control line is not present, the test is considered invalid.
Wyniki
Characterization of colloidal gold
The prepared colloidal gold solutions were claret red. TEM analyses were used to determine the morphology and shape of AuNPs (Figure 2A-D). Figure 2A and Figure 2B reveal that the particles are polyhedral in shape and uniformly distributed. The average diameter of AuNPs was found to be approximately 14 nm (Figure 2C). A high-reso...
Dyskusje
In this work, we present a protocol for the preparation of mAbs against natural product-derived small molecules. The essential steps and the matters needing attention in the procedure have been outlined, and we have demonstrated the utility of this protocol using the small molecule SSD as an example. Example spectra, TEM images, quantitative results and methodological investigations are shown in representative data. Hence, we have demonstrated that the colloidal gold production, AuNP-mAb conjugation and strip assembly st...
Ujawnienia
The authors have nothing to disclose.
Podziękowania
This work was supported by the Special Funds for Fundamental Research Funds of institutions of higher-learning affiliated with central departments. We appreciate the support of Classical Prescription Basic Research Team of the Beijing University of Chinese Medicine.
Materiały
| Name | Company | Catalog Number | Comments |
| Chloroauric acid solution (HAuCl4) | Tianjin Fu Chen Chemical Reagents Factory | JY-SJ102 | |
| bovine serum albumin | AMRESCO | 332 | |
| centrifuge tube 15 mL | Corning | 430645 | |
| centrifuge tube 50 mL | Corning | 430828 | |
| ELISA plates, 96 well | NUNC | 655101 | |
| Filter paper | Sinopharm | H5072 | |
| Glass fibre membranes | Jieyi | XQ-Y6 | |
| goat-anti-mouse IgG antibody | applygen | C1308 | |
| Nitrocellulose membranes | Millipore | millipore 180 | |
| ovalbumin | Beijing BIODEE | 5008-25g | |
| PEG20000 | Sigma Aldrich | RNBC6325 | |
| Pipette 10mL | COSTAR | 4488 | |
| Pipette 25mL | FALCON | 357525 | |
| semi-rigid PVC sheets | Jieyi | JY-C104 | |
| Sodium citrate | Beijing Chemical Works | C1034 | |
| sodium periodate | Sinopharm Chemical | BW-G0008 | |
| Sulfo-GMBS | Perbio Science Germany | 22324 | |
| TipOne Tips 1,000 µL | Starlab | S1111-2021 | |
| Tris-HCl | Solarbio | 77-86-1 | |
| TWEEN 20 | Solarbio | 9005-64-5 |
Odniesienia
- Huang, X., et al. Membrane-based lateral flow immunochromatographic strip with nanoparticles as reporters for detection: A review. Biosensors and Bioelectronics. 75, 166-180 (2016).
- Chang, H. -. F., Wang, J. -. Q., Wang, B., Deng, A. -. P. An immune chromatographic assay for rapid and simultaneous detection of levonorgestrel and methylprednisolone in water samples. Chinese Chemical Letters. 24 (10), 937-940 (2013).
- Lai, J. J., Stayton, P. S. Improving lateral-flow immunoassay (LFIA) diagnostics via biomarker enrichment for mHealth. Methods in Molecular Biology. 1256, 71-84 (2015).
- Zhang, M. Z., et al. Development of a colloidal gold-based lateral-flow immunoassay for the rapid simultaneous detection of clenbuterol and ractopamine in swine urine. Analytical & Bioanalytical Chemistry. 395 (8), 2591-2599 (2009).
- Kranthi, K. R., et al. Development of a colloidal-gold based lateral-flow immunoassay kit for 'quality-control' assessment of pyrethroid and endosulfan formulations in a novel single strip format. Crop Protection. 28 (5), 428-434 (2009).
- Qian, K., et al. Development and evaluation of an immunochromatographic strip for rapid detection of capsid protein antigen p27 of avian leukosis virus. Journal of Virological Methods. (221), 115-118 (2015).
- Guo, H., et al. Lateral flow immunoassay devices for testing saliva and other liquid samples and methods of use of same. US Patent. , (2003).
- Miočević, O., et al. Quantitative Lateral Flow Assays for Salivary Biomarker Assessment: A Review. Frontiers in Public Health. 5, 1-13 (2017).
- Lisa, M., et al. Gold nanoparticles based dipstick immunoassay for the rapid detection of dichlorodiphenyltrichloroethane: an organochlorine pesticide. Biosensors and Bioelectronics. 25 (1), 224-227 (2009).
- Zhang, Z., et al. Monoclonal Antibody-Europium Conjugate-Based Lateral Flow Time-Resolved Fluoroimmunoassay for Quantitative Determination of T-2 Toxin in Cereals and Feed. Analytical Methods. 7 (6), 2822-2829 (2015).
- Shen, H., et al. Facile synthesis of high-quality CuInZnxS2+x core/shell nanocrystals and their application for detection of C-reactive protein. Journal of Materials Chemistry. 22 (35), 18623-18630 (2012).
- Xiang, T., et al. A novel double antibody sandwich-lateral flow immunoassay for the rapid and simple detection of hepatitis C virus. International Journal of Molecular Medicine. 30 (5), 1041-1047 (2012).
- Yang, Q., et al. Quantum dot-based immunochromatography test strip for rapid, quantitative and sensitive detection of alpha fetoprotein. Biosensors & Bioelectronics. 30 (1), 145 (2011).
- Song, L. W., et al. Rapid fluorescent lateral-flow immunoassay for hepatitis B virus genotyping. Analytical Chemistry. 87, 5173-5180 (2015).
- Zhang, Y., et al. Quantum dot-based lateral-flow immunoassay for rapid detection of rhein using specific egg yolk antibodies. Artificial Cells, Nanomedicine, and Biotechnology. 1, (2017).
- Qu, H., et al. Rapid Lateral-Flow Immunoassay for the Quantum Dot-based Detection of GsRerarin. Biosensors and Bioelectronics. 81, 358-362 (2016).
- Li, Z., et al. Development and Clinical Application of a Rapid IgM-IgG Combined Antibody Test for SARS-CoV-2 Infection Diagnosis. Journal of Medical Virology. 92 (9), (2020).
- Xiaomei, L., Jing, W., Ya, Z. The clinical application value analysis of the 2019-coronary virus disease was analyzed by the whole blood Sars-COV 2 specific antibody detection. Natural Science Edition. 42, (2020).
- Zhang, Y., et al. Generation of Monoclonal Antibodies Against Natural Products. Journal of Visualized Experiments. , e57116 (2019).
- Sai, J., et al. Development of an Enzyme-Linked Immunosorbent Assay and Immunoaffinity Column Chromatography for Saikosaponin d Using an Anti-Saikosaponin d Monoclonal Antibody. Planta Medica. 82, 432-439 (2016).
- Yue, Z., et al. A Highly Sensitive Immunochromatographic Strip Test for Rapid and Quantitative Detection of Saikosaponin d. Molecules. 23 (2), 338 (2018).
- Qu, H., et al. Rapid Lateral-Flow Immunoassay for the Quantum Dot-based Detection of Puerarin. Biosensors and Bioelectronics. 81, 358-362 (2016).
- Zhang, Y., et al. Quantum dot-based lateral-flow immunoassay for rapid detection of rhein using specific egg yolk antibodies. Artificial Cells, Nanomedicine, and Biotechnology. 1, (2017).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone