Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Investigating Cardiac Metabolism in the Isolated Perfused Mouse Heart with Hyperpolarized [1-13C]Pyruvate and 13C/31P NMR Spectroscopy
W tym Artykule
Podsumowanie
We describe an experimental setup for administrating hyperpolarized 13C-labeled metabolites in continuous perfusion mode to an isolated perfused mouse heart. A dedicated 13C-NMR acquisition approach enabled the quantification of metabolic enzyme activity in real-time, and a multiparametric 31P-NMR analysis enabled the determination of the tissue ATP content and pH.
Streszczenie
Metabolism is the basis of important processes in cellular life. Characterizing how metabolic networks function in living tissues provides crucial information for understanding the mechanism of diseases and designing treatments. In this work, we describe procedures and methodologies for studying in-cell metabolic activity in a retrogradely perfused mouse heart in real-time. The heart was isolated in situ, in conjunction with cardiac arrest to minimize the myocardial ischemia and was perfused inside a nuclear magnetic resonance (NMR) spectrometer. While in the spectrometer and under continuous perfusion, hyperpolarized [1-13C]pyruvate was administered to the heart, and the subsequent hyperpolarized [1-13C]lactate and [13C]bicarbonate production rates served to determine, in real-time, the rates of lactate dehydrogenase and pyruvate dehydrogenase production. This metabolic activity of hyperpolarized [1-13C]pyruvate was quantified with NMR spectroscopy in a model free-manner using the product selective saturating-excitations acquisition approach. 31P spectroscopy was applied in between the hyperpolarized acquisitions to monitor the cardiac energetics and pH. This system is uniquely useful for studying metabolic activity in the healthy and diseased mouse heart.
Wprowadzenie
Alterations in cardiac metabolism are associated with a variety of cardiomyopathies and often form the basis of the underlying pathophysiological mechanisms1. However, there are numerous obstacles to studying metabolism in living tissues, as most biochemical assays require the homogenization of the tissue and cell lysis and/or radioactive tracing. Therefore, there is a pressing need for new tools to investigate myocardial metabolism in living tissues. Magnetic resonance (MR) of hyperpolarized 13C-labeled substrates allows for real-time measurements of metabolism in living tissues2, without the use of ionizing radiation, by increasing the MR signal-to-noise (SNR) ratio of the labeled site(s) by several orders of magnitude3. Here, we describe an experimental setup, an acquisition approach, and an analytical approach for studying the rapid metabolism in the isolated mouse heart and, in parallel, present indicators of general tissue energetics and acidity. The cardiac pH is a valuable indicator, as the acid-base balance is disrupted in the early stages of cardiac diseases and conditions such as myocardial ischemia, maladaptive hypertrophy, and heart failure6.
Hyperpolarized [1-13C]lactate and [13C]bicarbonate production from hyperpolarized [1-13C]pyruvate helps in determining the production rates of lactate dehydrogenase (LDH) and pyruvate dehydrogenase (PDH). Most of the previous studies performed using hyperpolarized substrates in the isolated rodent heart either used complex kinetic models to derive the enzymatic activity of LDH and PDH, or reported the signal intensity ratios of the hyperpolarized product to a substrate without calculating the actual enzyme activity rates2,4,5,6,7,8,9,10,11,12,13,14. Here, we used the product selective saturating-excitations approach15, which allows for the monitoring of the enzyme activity in a model-free manner15,16. In this way, the absolute enzymatic rates (i.e., the number of moles of product produced per unit of time) were determined. 31P spectroscopy was utilized to observe the signals of inorganic phosphate (Pi), phosphocreatine (PCr), and adenosine triphosphate (ATP). A multi-parametric analysis was used to characterize the pH distribution of the heart, as demonstrated by the heterogeneous chemical shift in the Pi signal of the tissue.
The retrogradely perfused mouse heart (Langendorff heart)17,18,19 is an ex vivo model for the intact beating heart. In this model, the heart viability and pH are preserved for at least 80 min20, and it has shown potential for recovery following a prolonged ischemic injury21,22. Nevertheless, inadvertent variability during micro-surgery may lead to variability in the tissue viability across hearts. Previous studies have reported on the deterioration of this heart over time19; for example, a reduction in contractile function of 5%-10% per hour has been observed18. The adenosine triphosphate (ATP) signal has previously been shown to report on the myocardial energetic status and viability23. Here, we noted that the perfused heart may occasionally show unintentional variability in viability levels, as demonstrated by the ATP content, despite the fact that we had an uninterrupted perfusion and oxygen supply. We demonstrate here that normalizing the LDH and PDH rates to the ATP content of the heart reduces the inter-heart variability in these rates.
In the following protocol, we describe the surgical procedure used for heart cannulation, isolation, and consequent perfusion in the NMR spectrometer. Of note, other surgical approaches aimed at isolating and perfusing the mouse heart have been described before24,25.
The methodologies used for acquiring data related to enzymatic rates in the beating heart (using 13C spectroscopy and hyperpolarized [1-13C]pyruvate) and the heart's viability and acidity (using 31P NMR spectroscopy) are described as well. Finally, the analytical methodologies for determining metabolic enzyme activities and tissue viability and acidity are explained.
Protokół
The joint ethics committee (IACUC) of the Hebrew University and Hadassah Medical Center approved the study protocol for animal welfare (MD-19-15827-1).
1. Krebs-Henseleit buffer preparation
- A day before the experiment, prepare a modified version of the Krebs-Henseleit buffer (KHB)26. Initially, dissolve 118 mM NaCl, 4.7 mM KCl, 0.5 mM pyruvate, 1.2 mM MgSO4, 25 mM NaHCO3, and 1.2 mM KH2PO4 in double-distilled H2O.
- Bubble this mixture with 95%/5% O2/CO2 for 20 min, and then add 1.2 mM CaCl2.
- Adjust the pH of the buffer to 7.4 with HCl or NaOH.
- On the day of the experiment, add 10 mM glucose and 72 U/L insulin to the KHB prepared in step 1.2.
NOTE: Insulin is added to the perfusion buffer as described in the work of Kolwicz et al.26 and in agreement with previous studies reporting that insulin increases the contractile function27 and the intensity of the hyperpolarized [13C]bicarbonate signal28.
2. Perfusion system preparation
- Keep a reservoir of 200 mL of KHB in a water bath at 40 °C, and bubble with 95%/5% O2/CO2 at a flow rate of 4 L/min for 1 h prior to cardiac perfusion. Keep the buffer continuously bubbling with this gas mixture throughout the experiment.
- First, set the water bath to 40 °C. Insert the KHB reservoir. Use a peristaltic pump (see Table of Materials) and medical-grade extension tubes to recirculate the KHB between the buffer reservoir and the 10 mm NMR tube at a constant flow rate of 7.5 mL/min.
- Connect three platinum-cured silicone tubes (3 mm i.d.) to the pump (one inflow tube and two outflow tubes for the KH buffer). Insert the outflow and inflow lines into the heated KH buffer. Then, insert the oxygen line into the heated KH buffer.
- Use thin polyether ether ketone (PEEK, see Table of Materials) lines for the buffer and hyperpolarized agent to flow to and from the NMR tube within the spectrometer's bore.
- Ensure that the temperature is maintained at 37-37.5 °C. Follow the steps below.
- Wrap the inflow line (from the buffer reservoir to the NMR tube) with a heating tape set to 42 °C.
- Heat the NMR tube inside the spectrometer with a warm airflow that is regulated by the spectrometer.
- Use an NMR-compatible temperature sensor (see Table of Materials) to measure the temperature inside the NMR tube. The temperature is adjusted to 37-37.5 °C.
3. Calibration and preparation of the NMR spectrometer for acquisition
- On the day of the experiment, insert a 13C standard sample that contains 1,4-dioxane (Table of Materials) into the spectrometer, and tune and match the NMR probe for 13C. Then, obtain a spectrum showing the thermal equilibrium signal of 1,4-dioxane with a nutation angle of 90°.
- Now exchange the 13C standard sample with a 31P standard sample (Table of Materials), that contains 105 mM of ATP in D2O. Tune and match the NMR probe for 31P.
NOTE: A spectrum showing the thermal equilibrium phosphate signals is obtained with a nutation angle of 50°. - Insert the inflow line, the outflow line, and the temperature probe into a 10 mm NMR tube, and then insert the tube into the magnetic bore. Adjust the heating tape to 42 °C.
- Acquire a 31P NMR spectrum of the circulating KH buffer to be used in that experiment for 30 min, with a nutation angle of 50° and a TR of 1.1 s (1,640 acquisitions).
4. Animal preparation, surgical procedure, and perfusion of the heart in the NMR tube
- Anesthetize a male HSD:ICR (CD-1) mouse with 3.3% isoflurane in room air (Table of Materials) at 340 mL/min for 5 min using a gas anesthesia system (Table of Materials) in an induction chamber.
- Use nasal anesthesia for the maintenance of general anesthesia with 2.9% isoflurane.
NOTE: Care is taken to minimize pain and discomfort to the animal. - Secure the limbs of the animal with tape, ensure a negative pedal pain reflex, and then inject 300 IU of sodium heparin intraperitoneally.
- Wet the mouse's chest wall and abdomen thoroughly with 70% alcohol to ensure cleanliness and avoid hair contamination or obstruction during the surgical procedure.
- At 1 min after the heparin injection, cut the skin and muscle of the abdominal cavity with small scissors.
- Place the small curved-jaw hemostat-locking mosquito clamp between the xiphoid process and the chest skin to lift the chest and expose the diaphragm. Puncture and cut the right lobe of the diaphragm.
- Cut the chest across the midline, retract to the sides, and then remove.
- Inject the left ventricle of the heart with 200 IU of sodium heparin to prevent blood coagulation. Then, inject 0.1 mL of ice-cold 0.5 mol/L KCl to achieve cardiac arrest, as previously described25. Cardiac arrest is essential to be able to cannulate the heart.
- Identify the thymus, and remove it using scissors to expose the aorta. Remove residual rib cage tissue.
- Identify the aortic arch, and use curved forceps to place a loose knot with a 3-0 silk suture (Table of Materials) around the ascending aorta. Inject 3 mL of KHB into the left ventricle to remove blood clots from the aorta.
- Use curved forceps to retract the heart inferiorly for better visualization of the ascending aorta.
- Perform cannulation in situ with a 22 G intravenous catheter (Table of Materials). Apply cyanoacrylate adhesive in the cannulated region, and then perform double suture tying. Inject additional KH buffer into the heart, and verify that it flows through the cannulation tube.
- Remove the curved forceps. Disconnect the heart from the surrounding viscera, and perfuse it retrogradely with ice-cold KHB (4 °C) through the intravenous catheter.
- Connect the heart to the inflow line of the perfusion system via the intravenous catheter. Upon the initiation of perfusion with warm buffer (37-37.5 °C) at 7.5 mL/min, the heart begins beating spontaneously.
- Fix the NMR tube with the beating heart, outflow lines, and temperature probe, and insert into the bore of the spectrometer, making sure the heart is at the center of the NMR probe.
5. Acquiring data for cardiac energetics and pH
- Acquire 31P spectra for about 1 h with a flip angle of 50 ° and a TR of 1.1 s.
6. DNP spin polarization and dissolution
- Prepare a 28.5 mg formulation of [1-13C]pyruvate. This formulation consists of 11.1 mM to 14.0 mM OX063 radical in the neat acid.
- Prepare 4 mL of dissolution medium. The dissolution medium consists of TRIS-phosphate buffer, which contains 11.2 mM NaH2PO4, 38.8 mM Na2HPO4, 33 mM TRIS, and 2 mM HCl. This medium composition is adjusted such that upon the addition of 28.5 mg of [1-13C]pyruvic acid formulation to 4 mL of this buffer (in the dissolution phase), the pH of the resulting solution will be 7.4.
- Perform spin polarization and fast dissolution in a dissolution-DNP (dDNP) spin polarization device according to the manufacturer's instructions (Table of Materials). Apply microwave irradiation at a frequency of 94.110 GHz for the polarization of the [1-13C]pyruvic acid formulation at 1.45 K to 1.55 K for about 1.5 h.
- Quickly mix the 4 mL of hyperpolarized medium from the dDNP device with a well-oxygenated solution that complements the hyperpolarized dissolution medium to obtain a composition closely matching the perfusion medium.
NOTE: The final volume of the medium perfusing the heart during the hyperpolarized injections with 14 mM hyperpolarized [1-13C]pyruvate is 26 mL. The final composition of the injected medium (after mixing) contains 4.7 mM KCl, 1.2 mM MgSO4, 70 mM NaCl, 25 mM NaHCO3, 1.2 mM KH2PO4, 10 mM glucose, 1.2 mM CaCl2, and 72 U/L insulin. - Administer the hyperpolarized [1-13C]pyruvate-containing medium to the isolated heart using a continuous flow setup29.
NOTE: This is done to ensure that the heart perfusion is not disturbed at any point during the experiment and that the hyperpolarized medium is administered at a known rate and for a known duration.
7. Hyperpolarized 13C spectroscopy
- Acquire hyperpolarized 13C data using product-selective saturating-excitation pulses15 by applying 2.5 ms cardinal sine (Sinc) pulses, as previously described15,16. Selectively excite [1-13C]lactate and [13C]bicarbonate consecutively at 6 s intervals to obtain a 12 s interval for each metabolite.
- For [1-13C]lactate detection, center the selective Sinc pulse at the [1-13C]pyruvate hydrate frequency (179.4 ppm), which results in signal intensity ratio (lac) of 0.113 for the C1 signals of [1-13C]pyruvate to [1-13C]lactate.
- For [13C]bicarbonate detection, center the selective Sinc pulse at 157.7 ppm, which is 214 Hz down-field of the [13C]bicarbonate signal (161.1 ppm); this results in a signal intensity ratio (bic) of 0.139 for the C1signal of [1-13C]pyruvate to [13C]bicarbonate.
8. Determination of the tissue wet weight and volume
- At the end of the experiment, detach the heart from the perfusion system, and gently dry it with tissue paper. Subsequently, weigh the heart to obtain the tissue wet weight.
- Determine the volume of the heart using a density factor of 1.05 g/cm3, as determined previously for the mouse heart30.
9. ATP content quantification
- Integrate the γ-ATP signal of a single acquisition of the ATP standard sample and a 30 min acquisition (TR of 1.1 s and 1,640 acquisitions) of the isolated heart.
- Quantify the ATP content of the heart by comparing the integral of the heart's γ-ATP signal to that of the standard (Table of Materials), where the latter has a known concentration (105 mM), and correct for the number of acquisitions and relaxation effects.
10. Resolving the Pi signal of the heart
NOTE: In order to evaluate the tissue pH, it is first necessary to deconvolve the heart's Pi signal from that of the total Pi signal (Pit). This is done by omitting the signal of the KHB Pi (PiKH) from that of the Pit.
- In a 31P spectrum of KHB that shows a single Pi signal (PiKH, Figure 1A), fit the PiKH signal to a Lorentzian function using Excel (Table of Materials).
- The volume visible to the NMR probe (Vp, 1.375 mL), contains more KHB when the sample tube does not contain the heart. To correct for this filling effect, calculate the attenuated buffer signal (Pib) using Eq. 1A.
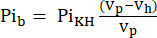 Eq. 1A
Eq. 1A
where Vh is the heart's volume, as determined in step 8. - Subtract this signal from the Pit according to Eq. 1B to obtain the Pi signal arising solely from the perfused heart (Pih, Figure 1B).
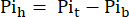 Eq. 1B
Eq. 1B
11. Multi-parametric pH analysis
- Perform the conversion of the Pih signal chemical shift distribution to the pH with reference to the chemical shift of PCr using Eq. 231.
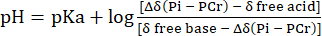 Eq. 2
Eq. 2
where Δδ is the chemical shift difference, pKa is 6.72, δ free base is 5.69, and δ free acid is 3.27, as previously described31. - Correct the resulting pH distribution curve for the non-linearity between the Pih chemical shift scale and the pH scale according to Lutz et al.32. A typical pH distribution resulting from this calculation is presented in Figure 1C.
- Analyze the tissue pH distribution with a multi-parametric approach using seven statistical parameters following the work of Lutz et al.32. Four of these parameters are presented here as they appear to be the most descriptive of tissue pH: 1) global maximum pH; 2) weighted mean pH; 3) weighted median pH; and 4) skewness of the pH plot (Figure 1C).
12. Calculation of the LDH and PDH activities
NOTE: The production rates of the hyperpolarized metabolites [1-13C]lactate and [13C]bicarbonate are used to calculate the LDH and PDH activities, respectively. In the product selective saturating-excitation approach15, only newly synthesized hyperpolarized metabolites are detected by each selective excitation.
- Use the hyperpolarized [1-13C]pyruvate signal as a reference to determine the corresponding metabolite production level.
- During the perfusion with the hyperpolarized medium, the [1-13C]pyruvate concentration in the NMR tube increases (wash-in), then plateaus (at a maximal concentration of 14 mM), and then decreases (wash-out).
- To identify the time points at which the pyruvate concentration reached a constant level (plateau), correct the [1-13C]pyruvate signal for signal decay resulting from T1 relaxation and RF pulsation using the effective relaxation constant, Teff.
- For each injection, define the Teff based on its ability to correct the [1-13C]pyruvate decay curve to show this flow dynamics (Eq.3).
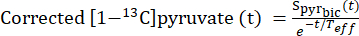 Eq. 3
Eq. 3
where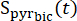 is the [1-13C]pyruvate signal that was acquired during the [13C]bicarbonate acquisition. The average Teff in the experiments described herein was found to be 35.8 s ± 2.3 s (n = 5 hearts).
is the [1-13C]pyruvate signal that was acquired during the [13C]bicarbonate acquisition. The average Teff in the experiments described herein was found to be 35.8 s ± 2.3 s (n = 5 hearts). - Select the data points in which the concentration is within 10% of the maximal corrected [1-13C]pyruvate signal for further analysis.
- Use the corresponding data of [1-13C]lactate and [13C]bicarbonate production for the time points selected in step 12.1 for the calculation of the metabolite production rates using Eq. 4A and Eq. 4B, provided that the SNR of the metabolite signal is larger than 2 (threshold for analysis). A typical example of such a selection of time points is shown in Figure 2B (highlighted temporal window).
- Calculate the production rates of each of the selected data points using Eq. 4A and Eq. 4B:
 Eq. 4A
Eq. 4A
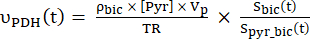 Eq. 4B
Eq. 4B
where and
and 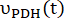 are the production rates of [1-13C]lactate or [13C]bicarbonate at each time point, respectively.
are the production rates of [1-13C]lactate or [13C]bicarbonate at each time point, respectively.  and
and 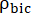 are factors that represent the relative excitation of [1-13C]pyruvate and the products [1-13C]lactate or [13C]bicarbonate, respectively. These factors were determined previously to be 0.113 and 0.139, respectively29. Vp is the volume that is detected by the NMR probe (1.375 mL), TR denotes the time interval between two consecutive product selective saturating excitations (12 s for each product),
are factors that represent the relative excitation of [1-13C]pyruvate and the products [1-13C]lactate or [13C]bicarbonate, respectively. These factors were determined previously to be 0.113 and 0.139, respectively29. Vp is the volume that is detected by the NMR probe (1.375 mL), TR denotes the time interval between two consecutive product selective saturating excitations (12 s for each product), 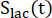 and
and 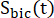 are the signals of [1-13C]lactate and [13C]bicarbonate, respectively, and
are the signals of [1-13C]lactate and [13C]bicarbonate, respectively, and 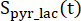 and
and  are the signals of [1-13C]pyruvate that were acquired during the [1-13C]lactate and [13C]bicarbonate excitations, respectively. [Pyr] is the [1-13C]pyruvate concentration, which was 14 mM during the plateau phase.
are the signals of [1-13C]pyruvate that were acquired during the [1-13C]lactate and [13C]bicarbonate excitations, respectively. [Pyr] is the [1-13C]pyruvate concentration, which was 14 mM during the plateau phase.
- Determine the rate for each point and then average per hyperpolarized injection.
Wyniki
The 31P spectra recorded from a mouse heart perfused with KHB and from the buffer alone are shown in Figure 1A. The signals of α-, β-, and γ-ATP, PCr, and Pi were observed in the heart. The Pi signal was composed of two main components: in the higher field (left side of the signal), the Pi signal was mostly due to the KHB at a pH of 7.4; in the lower field (right side of the signal), the Pi signal was broader and less homogeneous due to the more acidic environment. ...
Dyskusje
We demonstrate an experimental setup that is designed to investigate hyperpolarized [1-13C]pyruvate metabolism, tissue energetics, and pH in an isolated mouse heart model.
The critical steps within the protocol are as follows: 1) ensuring that the pH of the buffer is 7.4; 2) ensuring that all components of the buffer are included; 3) avoiding blood clotting in the cardiac vessels by heparin injections; 4) avoiding ischemic damage to the heart by reducing the metabolic activity (KCl ...
Ujawnienia
There are no disclosures.
Podziękowania
This project received funding from the Israel Science Foundation under grant agreement No. 1379/18; the Jabotinsky Scholarship of the Israeli Ministry of Science and Technology for Applied and Engineering Sciences for Direct PhD Students No. 3-15892 for D.S.; and the European Union's Horizon 2020 research and innovation program under grant agreement No. 858149 (AlternativesToGd).
Materiały
| Name | Company | Catalog Number | Comments |
| Equipment | |||
| HyperSense DNP Polariser | Oxford Instruments | 52-ZNP91000 | HyperSense, 3.35 T, preclinical dissolution-DNP hyperpolarizer |
| NMR spectrometer | RS2D | NMR Cube, 5.8 T, equipped with a 10 mm broad-band probe | |
| Peristaltic pump | Cole-Parmer | 07554-95 | |
| Temperature probe | Osensa | FTX-100-LUX+ | NMR compatible temprature probe |
| Somnosuite low-flow anesthesia system | Kent Scientific | ||
| Lines, tubings, suture | |||
| Platinum cured silicone tubes | Cole-Parmer | HV-96119-16 | L/S 16 I.D. 3.1 mm |
| Thin polyether ether ketone (PEEK) lines | Upchurch Scientific | id. 0.040” | |
| Intravenous catheter | BD Medical | 381323 | 22 G |
| Silk suture | Ethicon | W577H | Wire diameter of 3-0 |
| Chemicals and pharmaceuticals | |||
| [1-13C]pyruvic acid | Cambridge Isotope Laboratories | CLM-8077-1 | |
| Calcium chloride | Sigma-Aldrich | 21074 | CAS: 10043-52-4 |
| D-(+)-Glucose | Sigma-Aldrich | G7528 | CAS: 50-99-77 |
| Heparin sodium | Rotexmedica | HEP5A0130C0160 | |
| Hydrochloric acid 37% | Sigma-Aldrich | 258148 | CAS: 7647-01-0 |
| Insulin aspart (NovoLog) | Novo Nordisk | ||
| Isoflurane | Terrel | ||
| Magnesium Sulfate | Sigma-Aldrich | 793612 | CAS: 7487-88-9 |
| Potassium chloride | Sigma-Aldrich | P4504 | CAS: 7447-40-7 |
| Potassium phosphate monobasic | Sigma-Aldrich | P9791 | CAS: 7778-77-0 |
| Sodium bicarbonate | Gadot Group | CAS: 144-55-8 | |
| Sodium chloride | Sigma-Aldrich | S9625 | CAS: 7647-14-5 |
| Sodium hydroxide | Sigma-Aldrich | 655104 | CAS: 1310-73-2 |
| Sodium phosphate dibasic | Sigma-Aldrich | S7907 | CAS: 7558-79-4 |
| Sodium phosphate monobasic dihydrate | Merck | 6345 | CAS: 13472-35-0 |
| TRIS (biotechnology grade) | Amresco | 0826 | CAS: 77-86-1 |
| Trityl radical OX063 | GE Healthcare AS | NC100136 | OX063 |
| NMR standards | |||
| 13C standard sample | Cambridge Isotope Laboratories | DLM-72A | 40% p-dioxane in benzene-D6 |
| 31P standard sample | Made in house | 105 mM ATP and 120 mM phenylphosphonic acid in D2O | |
| Software | |||
| Excel 2016 | Microsoft | ||
| MNova | Mestrelab Research |
Odniesienia
- Aquaro, G. D., Menichetti, L. Hyperpolarized 13C-magnetic resonance spectroscopy: Are we ready for metabolic imaging. Circulation. Cardiovascular Imaging. 7 (6), 854-856 (2014).
- Schroeder, M. A., et al. Real-time assessment of Krebs cycle metabolism using hyperpolarized 13C magnetic resonance spectroscopy. FASEB Journal. 23 (8), 2529-2538 (2009).
- Ardenkjaer-Larsen, J. H., et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proceedings of the National Academy of Sciences of the United States of America. 100 (18), 10158-10163 (2003).
- Merritt, M. E., et al. Hyperpolarized C-13 allows a direct measure of flux through a single enzyme-catalyzed step by NMR. Proceedings of the National Academy of Sciences of the United States of America. 104 (50), 19773-19777 (2007).
- Ball, D. R., et al. Hyperpolarized butyrate: A metabolic probe of short chain fatty acid metabolism in the heart. Magn Reson Med. (5), 1663-1669 (2014).
- Khemtong, C., Carpenter, N. R., Lumata, L. L., et al. Hyperpolarized 13C NMR detects rapid drug-induced changes in cardiac metabolism. Magnetic Resonance in Medicine. 74 (2), 312-319 (2015).
- Mariotti, E., et al. Modeling non-linear kinetics of hyperpolarized [1-13C] pyruvate in the crystalloid-perfused rat heart. NMR in Biomedicine. 29 (4), 377-386 (2016).
- Moreno, K. X., Sabelhaus, S. M., Merritt, M. E., Sherry, A. D., Malloy, C. R. Competition of pyruvate with physiological substrates for oxidation by the heart: implications for studies with hyperpolarized [1-13C]pyruvate. American Journal of Physiology-Heart and Circulatory Physiology. 298 (5), H1556-H1564 (2010).
- Purmal, C., et al. Propionate stimulates pyruvate oxidation in the presence of acetate. American Journal of Physiology-Heart and Circulatory Physiology. 307 (8), H1134-H1141 (2014).
- Weiss, K., et al. Developing hyperpolarized 13C spectroscopy and imaging for metabolic studies in the isolated perfused rat heart. Applied Magnetic Resonance. 43 (1), 275-288 (2012).
- Merritt, M. E., Harrison, C., Storey, C., Sherry, A. D., Malloy, C. R. Inhibition of carbohydrate oxidation during the first minute of reperfusion after brief ischemia: NMR detection of hyperpolarized 13CO2and H13CO3. Magnetic Resonance in Medicine. 60 (5), 1029-1036 (2008).
- Schroeder, M. A., et al. Measuring intracellular pH in the heart using hyperpolarized carbon dioxide and bicarbonate: a 13C and 31P magnetic resonance spectroscopy study. Cardiovascular Research. 86 (1), 82-91 (2010).
- Ball, D. R., et al. Metabolic imaging of acute and chronic infarction in the perfused rat heart using hyperpolarised [1-13C]pyruvate. NMR in Biomedicine. 26 (11), 1441-1450 (2013).
- Atherton, H. J., et al. Role of PDH inhibition in the development of hypertrophy in the hyperthyroid rat heart: a combined magnetic resonance imaging and hyperpolarized magnetic resonance spectroscopy study. Circulation. 123 (22), 2552-2561 (2011).
- Harris, T., et al. Hyperpolarized product selective saturating-excitations for determination of changes in metabolic reaction rates in real-time. NMR in Biomedicine. 33 (2), e4189 (2020).
- Shaul, D., et al. Correlation between lactate dehydrogenase/pyruvate dehydrogenase activities ratio and tissue pH in the perfused mouse heart: A potential noninvasive indicator of cardiac pH provided by hyperpolarized magnetic resonance. NMR in Biomedicine. 34 (2), e4444 (2021).
- Jian, Z., et al. In vivo cannulation methods for cardiomyocytes isolation from heart disease models. PLoS One. 11 (8), e0160605 (2016).
- Sutherland, F. J., Hearse, D. J. The isolated blood and perfusion fluid perfused heart. Pharmacological Research. 41 (6), 613-627 (2000).
- Lateef, R., Al-Masri, A., Alyahya, A. Langendorff's isolated perfused rat heart technique: A review. International Journal of Basic and Clinical Pharmacology. 4, 1314-1322 (2015).
- Cross, H. R., Radda, G. K., Clarke, K. The role of Na+/K+ ATPase activity during low-flow ischemia in preventing myocardial injury - A 31P, 23Na and 87Rb NMR spectroscopic study. Magnetic Resonance in Medicine. 34 (5), 673-685 (1995).
- Cross, H. R., Clarke, K., Opie, L. H., Radda, G. K. Is lactate-induced myocardial ischaemic injury mediated by decreased pH or increased intracellular lactate. Journal of Molecular and Cellular Cardiology. 27 (7), 1369-1381 (1995).
- Clarke, K., O'Connor, A. J., Willis, R. J. Temporal relation between energy metabolism and myocardial function during ischemia and reperfusion. American Journal of Physiology. 253 (2), H412-H421 (1987).
- Yabe, T., Mitsunami, K., Inubushi, T., Kinoshita, M. Quantitative measurements of cardiac phosphorus metabolites in coronary artery disease by 31P magnetic resonance spectroscopy. Circulation. 92 (1), 15-23 (1995).
- Bakrania, B., Granger, J. P., Harmancey, R. Methods for the determination of rates of glucose and fatty acid oxidation in the isolated working rat heart. Journal of Visualized Experiments. (115), e54497 (2016).
- Cordeiro, B., Clements, R. Murine isolated heart model of myocardial stunning associated with cardioplegic arrest. Journal of Visualized Experiments. (102), e52433 (2015).
- Kolwicz, S. C., Tian, R. Assessment of cardiac function and energetics in isolated mouse hearts using 31P NMR spectroscopy. Journal of Visualized Experiments. (42), e2069 (2010).
- Nakadate, Y., et al. Glycemia and the cardioprotective effects of insulin pre-conditioning in the isolated rat heart. Cardiovascular Diabetology. 16 (1), 43 (2017).
- Lauritzen, M. H., et al. Enhancing the C-13 bicarbonate signal in cardiac hyperpolarized 1-C-13 pyruvate MRS studies by infusion of glucose, insulin and potassium. NMR in Biomedicine. 26 (11), 1496-1500 (2013).
- Adler-Levy, Y., et al. In-cell determination of lactate dehydrogenase activity in a luminal breast cancer model - ex vivo investigation of excised xenograft tumor slices using dDNP hyperpolarized [1-13C]pyruvate. Sensors. 19 (9), 2089 (2019).
- Young, A. A., Barnes, H., Davison, D., Neubauer, S., Schneider, J. E. Fast left ventricular mass and volume assessment in mice with three-dimensional guide-point modeling. Journal of Magnetic Resonance Imaging. 30 (3), 514-520 (2009).
- Bailey, I. A., Williams, S. R., Radda, G. K., Gadian, D. G. Activity of phosphorylase in total global ischaemia in the rat heart. A phosphorus-31 nuclear-magnetic-resonance study. Biochemical Journal. 196 (1), 171-178 (1981).
- Lutz, N. W., Le Fur, Y., Chiche, J., Pouyssegur, J., Cozzone, P. J. Quantitative in vivo characterization of intracellular and extracellular pH profiles in heterogeneous tumors: A novel method enabling multiparametric pH analysis. Cancer Research. 7 (15), 4616-4628 (2013).
- Harris, T., Gamliel, A., Sosna, J., Gomori, J. M., Katz-Brull, R. Impurities of [1-13C]pyruvic acid and a method to minimize their signals for hyperpolarized pyruvate metabolism studies. Applied Magnetic Resonance. 49 (10), 1085-1098 (2018).
- Cunningham, C. H., et al. Hyperpolarized 13C metabolic MRI of the human heart initial experience. Circulation Research. 119 (11), 1177-1182 (2016).
- Kurhanewicz, J., et al. Hyperpolarized 13C MRI: Path to clinical translation in oncology. Neoplasia. 21 (1), 1-16 (2019).
- Miloushev, V. Z., et al. Metabolic imaging of the human brain with hyperpolarized 13C pyruvate demonstrates 13C lactate production in brain tumor patients. Cancer Research. 78 (14), 3755-3760 (2018).
- Park, I., et al. Development of methods and feasibility of using hyperpolarized carbon-13 imaging data for evaluating brain metabolism in patient studies. Magnetic Resonance in Medicine. 80 (3), 864-873 (2018).
- Grist, J. T., et al. Quantifying normal human brain metabolism using hyperpolarized [1-13C]pyruvate and magnetic resonance imaging. Neuroimage. 189, 171-179 (2019).
- Nelson, S. J., et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-C]pyruvate. Science Translational Medicine. 5 (198), (2013).
- Stødkilde-Jørgensen, H., et al. Pilot study experiences with hyperpolarized [1-13C]pyruvate MRI in pancreatic cancer patients. Journal of Magnetic Resonance Imaging. 51 (3), 961-963 (2019).
- Autry, A. W., et al. Measuring tumor metabolism in pediatric diffuse intrinsic pontine glioma using hyperpolarized carbon-13 MR metabolic imaging. Contrast Media and Molecular Imaging. 2018, 3215658 (2018).
- Chung, B. T., et al. First hyperpolarized [2-13C]pyruvate MR studies of human brain metabolism. Journal of Magnetic Resonance. 309, 106617 (2019).
- Rider, O. J., et al. Noninvasive in vivo assessment of cardiac metabolism in the healthy and diabetic human heart using hyperpolarized 13C MRI. Circulation Research. 126 (6), 725-736 (2020).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone