Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Enhanced Oil Recovery using a Combination of Biosurfactants
W tym Artykule
Podsumowanie
We illustrate the methods involved in screening and identification of the biosurfactant producing microbes. Methods for chromatographic characterization and chemical identification of the biosurfactants, determining the industrial applicability of the biosurfactant in enhancing residual oil recovery are also presented.
Streszczenie
Biosurfactants are surface-active compounds capable of reducing the surface tension between two phases of different polarities. Biosurfactants have been emerging as promising alternatives to chemical surfactants due to less toxicity, high biodegradability, environmental compatibility and tolerance to extreme environmental conditions. Here, we illustrate the methods used for screening of microbes capable of producing biosurfactants. The biosurfactant producing microbes were identified using drop collapse, oil spreading, and emulsion index assays. Biosurfactant production was validated by determining the reduction in surface tension of the media due to growth of the microbial members. We also describe the methods involved in characterization and identification of biosurfactants. Thin layer chromatography of the extracted biosurfactant followed by differential staining of the plates was performed to determine the nature of the biosurfactant. LCMS, 1H NMR, and FT-IR were used to chemically identify the biosurfactant. We further illustrate the methods to evaluate the application of the combination of produced biosurfactants for enhancing residual oil recovery in a simulated sand pack column.
Wprowadzenie
Biosurfactants are the amphipathic surface-active molecules produced by microorganisms that have the capacity to reduce the surface and the interfacial tension between two phases1. A typical biosurfactant contains a hydrophilic part that is usually composed of a sugar moiety or a peptide chain or hydrophilic amino acid and a hydrophobic part that is made up of a saturated or unsaturated fatty acid chain2. Due to their amphipathic nature, biosurfactants assemble at the interface between the two phases and reduce the interfacial tension at the boundary, which facilitates the dispersion of one phase into the other1,3. Various types of biosurfactants that have been reported so far include glycolipids in which carbohydrates are linked to long chain aliphatic or hydroxy-aliphatic acids via ester bonds (e.g., rhamnolipids, trehalolipids and sophorolipids), lipopeptides in which lipids are attached to polypeptide chains (e.g., surfactin and lichenysin), and polymeric biosurfactants that are usually composed of polysaccharide- protein complexes (e.g., emulsan, liposan, alasan and lipomannan)4. Other types of biosurfactants produced by the microorganisms include fatty acids, phospholipids, neutral lipids, and particulate biosurfactants5. The most studied class of biosurfactants is glycolipids and among them most of the studies have been reported on rhamnolipids6. Rhamnolipids contain one or two molecules of rhamnose (which form the hydrophilic part) linked to one or two molecules of long chain fatty acid (usually hydroxy-decanoic acid). Rhamnolipids are primary glycolipids reported first from Pseudomonas aeruginosa7.
Biosurfactants have been gaining increasing focus as compared to their chemical counterparts due to various unique and distinctive properties that they offer8. These include higher specificity, lower toxicity, greater diversity, ease of preparation, higher biodegradability, better foaming, environmental compatibility and activity under extreme conditions9. Structural diversity of the biosurfactants (Figure S1) is another advantage that gives them an edge over the chemical counterparts10. They are generally more effective and efficient at lower concentrations as their critical micelle concentration (CMC) is usually several times lower than chemical surfactants11. They have been reported to be highly thermostable (up to 100 °C) and can tolerate higher pH (up to 9) and high salt concentrations (up to 50 g/L)12 thereby offer several advantages in industrial processes, which require exposure to extreme conditions13. Biodegradability and lower toxicity make them suitable for environmental applications such as bioremediation. Because of the advantages that they offer, they have been getting increased attention in various industries like food, agricultural, detergent, cosmetic and petroleum industry11. Biosurfactants have also gained a lot of attention in oil remediation for removal of petroleum contaminants and toxic pollutants14.
Here we report the production, characterization, and application of biosurfactants produced by Rhodococcus sp. IITD102, Lysinibacillus sp. IITD104, and Paenibacillus sp. IITD108. The steps involved in screening, characterization, and application of a combination of biosurfactants for enhanced oil recovery are outlined in Figure 1.
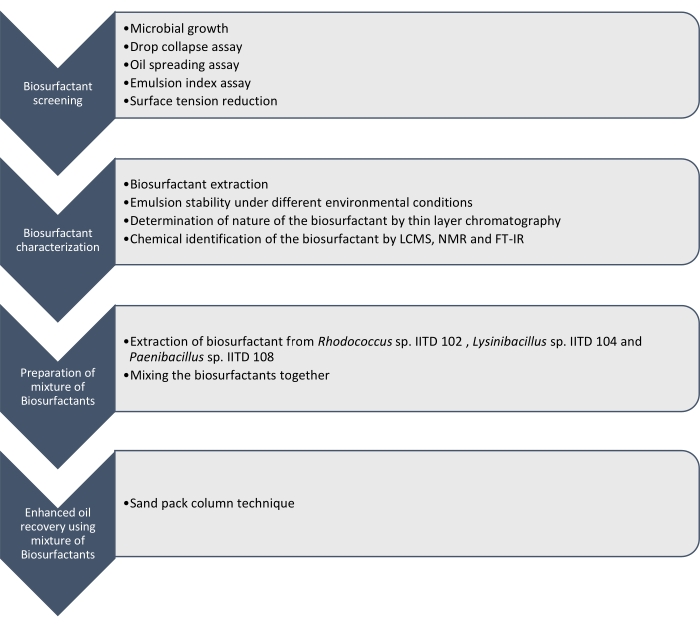
Figure 1: A method for enhanced oil recovery using a combination of Biosurfactants. The stepwise work flow is shown. The work was carried out in four steps. First the microbial strains were cultured and screened for the production of biosurfactant by various assays, which included drop collapse assay, oil spreading assay, emulsion index assay, and surface tension measurement. Then, the biosurfactants were extracted from the cell-free broth and their nature was identified using thin layer chromatography and they were further identified using LCMS, NMR, and FT-IR. In the next step, the extracted biosurfactants were mixed together and the potential of the resulting mixture for enhanced oil recovery was determined using the sand pack column technique. Please click here to view a larger version of this figure.
Screening of these microbial strains to produce biosurfactants was done by drop collapse, oil spreading, emulsion index assay and determination of reduction in the surface tension of the cell-free medium due to growth of the microbes. The biosurfactants were extracted, characterized, and chemically identified by LCMS, 1H NMR, and FT-IR. Finally, a mixture of biosurfactants produced by these microbes was prepared and was used to recover the residual oil in a simulated sand pack column.
The present study only illustrates the methods involved in screening, identification, structural characterization, and application of the biosurfactant combination on enhancing residual oil recovery. It does not provide a detailed functional characterization of the biosurfactants produced by the microbial strains15,16. Various experiments such as critical micelle determination, thermogravimetric analysis, surface wettability, and biodegradability are performed for detailed functional characterization of any biosurfactant. But since this paper is a methods paper, the focus is on screening, identification, structural characterization, and application of the biosurfactant combination on enhancing residual oil recovery; these experiments have not been included in this study.
Protokół
1. Growth of microbial strains
- Weigh 2 g of Luria Broth powder and add to 50 mL of distilled water in a 250 mL conical flask. Mix the contents until the powder dissolves completely and make up the volume to 100 mL using distilled water.
- Similarly, prepare two more flasks of 100 mL of Luria Broth and place cotton plugs on the neck of the flasks.
- Cover the cotton plugs with aluminum foil and autoclave the flasks for 15 min at 121 °C and 15 psi to sterilize the media.
- After autoclaving, let the media cool down to room temperature.
- For the preparation of primary culture of a strain, pick a single colony from an LA plate using an inoculation loop and inoculate in a test tube containing 5 mL of sterile Luria broth.
- Incubate the test tube overnight at 30 °C at 180 rpm.
- Inoculate the flasks containing 100 mL of autoclaved Luria Broth by adding 1 mL of overnight grown seed cultures to the flasks inside the laminar air flow cabinet.
- Incubate the flasks in a rotary incubator at 30 °C and 180 rpm for 7 days.
- After the completion of the incubation period, harvest the flasks and transfer the culture broth to the centrifuge tubes. Centrifuge the culture at 4,500 x g for 20 min in a refrigerated centrifuge at 4 °C.
- Gently, pour the cell free supernatant into a fresh beaker and use it in screening assays for biosurfactant production.
2. Screening assays for biosurfactant production
NOTE: In the following sections, commercial surfactant (Saponin) was used as a positive control while water and uninoculated media were used as a negative control.
- Drop collapse assay
- Take a clean glass slide and coat the surface of the slide with 200 µL of oil.
- Add 20 µL of the cell free supernatant to the center of the oil and leave it undisturbed for 2-3 min.
NOTE: If the drop collapses, score the supernatant positive for the presence of biosurfactant.
- Oil spreading assay
- Take 20 mL of double distilled water in a Petri plate (75 mm diameter) and add 200 µL of crude oil to the surface of the water.
- Add 20 µL of cell free supernatant to the center of the oil and leave it undisturbed for 1 min.
NOTE: If a clearing zone is formed because of oil displacement, score the supernatant positive for the presence of the biosurfactant.
- Emulsion index assay (E24 assay)
- Add 4 mL of petrol (gasoline) and cell-free supernatant each into a clean glass test tube.
- Vortex the mixture vigorously for 3 min and leave it undisturbed for the next 24 h.
- After 24 h, determine the E24 index as a percentage of the height of the emulsified layer (cm) with respect to the height of the entire liquid column (cm).
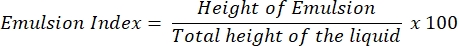
NOTE: If an emulsion (oil in water or water in oil) is observed after 24 h, the supernatant is likely to contain the biosurfactant.
- Surface tension measurement
NOTE: The surface tension was measured using the Du Noüy ring method17. The instrument used in this experiment (see Table of Materials) is very sensitive, so ensure proper cleaning of the glass vessel and the probe.- Turn on the system and double click on the associated software to open it.
- Clean the glass vessel with the liquid whose surface tension is to be determined.
- Add the liquid (40 mL) into the vessel and mount the vessel on the vessel holder.
- Unlock the probe holder and mount the probe on it. Now lock the probe holder by pressing the Lock button on the manual controller.
- Using the manual controller, adjust the height of the platform such that the probe is around 2-3 mm away from the surface of the liquid.
- Now use the software to measure the surface tension. Click on File located on the top left panel of the screen. Click on Open Workspace. A pop-up window will appear.
- Scroll down and double-click on the K100: Surface and Interfacial Tension icon.
- Now, click on the File icon located in the top-left corner of the screen. Click on New Database. Enter the name for saving the data and click on OK.
- Again, click on File > New Measurement > SFT > Ring. Enter the name for the measurement. Ensure that the configuration template shows SFT ring.
- Fill in the details in the Measurement Configuration window by selecting the probe and the vessel that is being used for the measurement. Also, fill in the details of the liquid and the gas phase.
NOTE: The liquid phase will be water, while the gas phase will be air. The density of the liquid phase is the density of the cell free supernatant. This can be determined by taking the weight of 50 mL of the liquid and calculating density as Kg/m3. - Now click on the Procedure tab and fill in the following details: Detection Speed: 6 mm/min, Detection Sensitivity: 0.005 g, Search Speed: 6 mm/min, Search Sensitivity: 0.005 g, Measuring Speed: 3 mm/min, Measuring Sensitivity: 0.001 g, Immersion Depth: 3 mm, Return Distance: 10%, correction: Harkins & Jordan, max values: 5. Click on OK.
- In the pop-up window, select the database to store data and click on OK.
NOTE: One can create a new database here or add new measurements to the existing data. - Now click on the Play button located on the top-center of the screen. The system will start executing the script. After the system stabilizes, it will detect the surface. Immerse the probe into the liquid, move the probe back and forth and detect the tension in the formed lamella.
- For obtaining the results, click on the Measurement icon on the middle-left side of the screen. Click on Data and note down the surface tension determined.
- After completion of the measurement, lower the height of the platform and unlock and unmount the probe and the vessel from the instrument.
NOTE: To determine the decrease in the surface tension due to biosurfactant production, uninoculated LB should be used as a control.
3. Biosurfactant extraction
- Adjust the pH of the cell free supernatant to 2 using 2 N HCl. Store the mixture at 4 °C overnight.
- Add equal volume of chloroform-methanol mixture (2:1) to the supernatant and mix vigorously for 20 min.
- Leave the mixture undisturbed for phase separation to occur.
- Remove the upper phase containing water and methanol and leave the lower phase containing the biosurfactant to evaporate in a fume hood.
- After evaporation of the organic phase, redissolve the honey-colored crude biosurfactant in 3 mL of chloroform and use this mixture for further identification and characterization of the biosurfactant.
4. Emulsion stability studies
- Emulsion stability at different temperatures
- Take 5 mL of cell free supernatants in different test tubes.
- Add 5 mL of petrol to each test tube and mix vigorously by vortexing for 3 min.
- Incubate the test tubes overnight in different water baths at different temperatures (30 °C, 40 °C, 50 °C, 60 °C, and 70 °C).
- After 24 h, estimate the emulsion indices as mentioned earlier.
- Emulsion stability at different pH values
- Take 5 mL of the cell free supernatant in clean test tubes.
- Adjust the pH of cell free supernatants (2, 4, 6, 8, and 10) using 1 N HCl and 1 N NaOH.
- Add an equal amount of petrol to the test tubes and mix vigorously by vortexing for 3 min.
- Leave the test tubes undisturbed at room temperature for 24 h.
- Estimate the emulsion index as mentioned earlier.
- Emulsion stability at different salt concentrations
- Take 5 mL of the cell free supernatant in clean test tubes.
- Add different amounts of salt (NaCl) to the supernatants (0 g/L, 5 g/L, 10 g/L, 20 g/L, 60 g/L and 80 g/L).
- Dissolve the salts in the cell free supernatants by vortexing for 3 min.
- Add an equal amount of petrol to the test tubes and mix vigorously by vortexing for 3 min.
- Leave the test tubes undisturbed at room temperature for 24 h.
- Estimate the emulsion index after 24 h.
5. Determining the nature of the biosurfactant
- TLC of the extracted biosurfactant
- Spot 20 µL of the biosurfactants on TLC plates. Spot 2 µL at one time.
- Spot the biosurfactants on three different TLC plates.
- Prepare a 100 mL mixture of the eluent containing chloroform:methanol (2:1) and add the eluent to the TLC chamber. Close the lid of the chamber and allow it to saturate for 20 min.
- After drying the plates, place the TLC plates inside the chamber saturated with a chloroform methanol mixture and run the TLC.
- After the eluent has reached the top of the TLC plate (1 cm away from the top), take the plates out and let it air dry.
- Staining for lipid detection
- Take a clean TLC chamber and add some (5-10) granules of iodine into the fresh chamber and saturate the chamber for 5 to 10 mins.
- Place the TLC plate inside the chamber and observe for the development of the yellow spots. If the spots appear, score the biosurfactant positive for the presence of the lipid component.
- Staining for peptide or amino acid detection
- Prepare a ninhydrin solution by dissolving 0.4 g of ninhydrin in 20 mL of butanol. Add 0.6 mL of 100% glacial acetic acid to the mixture.
- Spray the TLC plate with ninhydrin solution and let it air dry for 2 min. Heat the plate at 110 °C and observe the development of the color.
NOTE: If the blue spots appear, score the biosurfactant positive for the presence of any peptide chain or amino acid.
- Staining for carbohydrate detection
- Prepare a solution of p-anisaldehyde by adding 2 mL of p-anisaldehyde to 48 mL of glacial acetic acid containing 1 mL of H2SO4. Add 0.6 mL of acetic acid to the mixture.
- Spray the mixture evenly on a TLC plate and let it air dry for 2 min.
- Incubate the plate at 110 °C and monitor the development of spots.
NOTE: If the green or brown spots appear, score the biosurfactant positive for the presence of any carbohydrates.
6. Chemical identification of the biosurfactant
- LCMS of the biosurfactant
- Dissolve 25 mg of the extracted biosurfactant in 1 mL of chloroform.
- Perform LCMS (in a lock spray configuration with a reference scan frequency of 10 s) using a C18 column.
- Use chloroform:methanol (1:1) as a mobile phase and inject 2 µL of the sample into the column at a flow rate of 0.1 mL/min.
- Set the experimental parameters to: polarity: ES positive, capillary voltage: 3 kV, source temperature: 80 °C, desolvation temperature: 300 °C, desolvation gas flow rate: 7,000 L/h, and trap gas flow rate: 0.40 mL/min.
- Scan the ranges from 100 to 1,200 Da during a detection time of 20 mins and survey the ions in positive ES mode.
- Analyze the m/z values using any mass spectrometry quantitively software.
- For analysis, log into the software.
- Click on Batch Search and enter the list of the masses obtained. Survey the results in a positive charge mode and use M + H and M + Na as the adducts. Maintain the accuracy to 10 PPM and tick on Display Structure.
- Click on Search and from the list of compounds, select the one with the lowest PPM level.
- 1H NMR of the biosurfactant
NOTE: 1H NMR of the biosurfactant was performed using a 400 MHz NMR spectrometer (see Table of Materials).- Dissolve 5 mg of the biosurfactant in 1 mL of deuterated chloroform(CdCl3).
- Transfer the mixture to an NMR tube. Cap the tube properly and insert the tube in the spanner. Adjust the height of the tube using the adjuster tube.
- Place the tube along with the spanner in the NMR machine and follow the steps mentioned below to get an NMR spectrum.
- To select the sample tube type: sx N, where N is the position where the tube was placed (e.g., sx 13, if the tube was placed at the 13th position) in associated software.
- Type edc and press Enter to create a new folder where data can be stored.
- A pop up will appear. Select the solvent by clicking on CdCl 3 in the list and enter the name of the sample.
- To start the protocol, type "getprosol"; to lock the solvent, type "lock cdcl3".
- Type "topshim" to shim the sample, and finally type "rga;zgefp" for acquiring the data. This will start the protocol.
- After the spectra has been obtained, type "apk;abs n" and press enter for phase and baseline correction.
- To select primary peaks, type "pp" and press Enter. To select only intense peaks, enter "mi" and type in the intensity above which the peaks should be selected. The default value will be 0.2.
- To integrate the peaks, click on Integrate and place the cursor on the left side of the peak to be integrated and while holding the cursor click and drag the cursor around the peak.
- Save the data by clicking on File on the top-left corner, and then click on Save.
- The sample can be ejected from the machine by typing "sx ej".
- Analyze the peaks and determine the environment of the H atoms.
- Fourier Transform Infrared Spectroscopy of the biosurfactant
NOTE: FT-IR of extracted biosurfactant was performed using a commercially available spectrophotometer in ATR mode (see Table of Materials).- Turn the spectrophotometer on and check the purge, desiccant, and detector.
- To collect a spectrum, first collect the background spectrum without a sample in place.
- Take the extracted biosurfactant and dry it completely. Place the dried biosurfactant directly over the diamond crystal, apply pressure and press the ATR touch point.
- In the software, select the number of the scans (enter 30) and scan the spectrum from 400 cm-1 to 4,000 cm-1.
- Click on OK to add the sample spectrum to the spectral window.
- Click on Files > Save > Save As and enter the file name followed by extension .spa and click on OK.
7. Biosurfactant application (enhanced oil recovery)
NOTE: In this experiment, double distilled water was used as a negative control and 10% SDS, 10% Tween 80, and 10% commercial saponin were used as positive controls.
- Take the glass and seal the bottom outlet with glass wool and glass beads.
- Pack the column with sandy soil in such a way that some liquid can be added at the top of the soil and the flow through can be collected at the bottom. Mount the column on the holder and add some glass beads on top of the soil.
- Flood the column with 50 mL of brine solution and collect the flow through to determine the pore volume.
pore volume = volume of brine added on top - volume of flowthrough collected. - Remove the brine from the column by forcing crude oil to pass through it after adding from the top of the column. Collect the volume of the brine and oil coming out of the column to determine initial oil saturation volume. The volume of the brine released from the column will be the initial oil saturation volume or original oil in place.
- Leave the column undisturbed for 24 h.
- After 24 h, flood the column with 10 pore volumes of brine and collect the oil coming out of the column to estimate secondary oil recovery. The oil left in the column after secondary oil recovery corresponds to the residual oil.
- Prepare a mixture of biosurfactants by adding equal volumes of the extracted biosurfactant (extracted after step 3.5) to the glass beaker. Add the biosurfactants to the top of the column and incubate the column for 24 h.
- After 24 h, measure the amount of oil and water to determine additional or enhanced oil recovery. The volume of the oil released from the column will correspond to the residual oil recovered.
- Estimate enhanced oil recovery with the following equation:
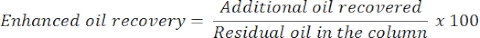
Wyniki
Three bacterial strains (Rhodococcus sp. IITD102, Lysinibacillus sp. IITD104, and Paenibacillus sp. IITD108) were screened for the production of biosurfactants by various assays, which included drop collapse assay, oil displacement assay, emulsion index assay, and surface tension reduction. Cell-free supernatants of all the three bacterial strains and a solution of chemical surfactant resulted in a drop collapse and, therefore, were scored positive for the presence of the biosurfactants (
Dyskusje
Biosurfactants are one of the most versatile group of biologically active components that are becoming attractive alternatives to chemical surfactants. They have a wide range of applications in numerous industries such as detergents, paints, cosmetics, food, pharmaceuticals, agriculture, petroleum, and water treatment due to their better wettability, lower CMC, diversified structure, and environmental friendliness18. This has led to an increased interest in discovering more microbial strains capab...
Ujawnienia
The authors declare no conflicts of interest.
Podziękowania
The authors would like to thank the Department of Biotechnology, Government of India, for financial support.
Materiały
| Name | Company | Catalog Number | Comments |
| 1 ml pipette | Eppendorf, Germany | G54412G | |
| 1H NMR | Bruker Avance AV-III type spectrometer,USA | ||
| 20 ul pipette | Thermo scientific, USA | H69820 | |
| Autoclave | JAISBO, India | Ser no 5923 | Jain Scientific |
| Blue flame burner | Rocker scientific, Taiwan | dragon 200 | |
| Butanol | GLR inovations, India | GLR09.022930 | |
| C18 column | Agilent Technologies, USA | 770995-902 | |
| Centrifuge | Eppendorf, Germany | 5810R | |
| Chloroform | Merck, India | 1.94506.2521 | |
| Chloroform-d | SRL, India | 57034 | |
| Falcon tubes | Tarsons, India | 546041 | Radiation sterilized polypropylene |
| FT-IR | Thermo Fisher Scientific, USA | Nicolet iS50 | |
| Fume hood | Khera, India | 47408 | Customied |
| glacial acetic acid | Merck, India | 1.93002 | |
| Glass beads | Merck, India | 104014 | |
| Glass slides | Polar industrial Corporation, USA | Blue Star | 75 mm * 25 mm |
| Glass wool | Merk, India | 104086 | |
| Hydrochloric acid | Merck, India | 1003170510 | |
| Incubator | Thermo Scientific, USA | MaxQ600 | Shaking incubator |
| Incubator | Khera, India | Sunbim | |
| Iodine resublimed | Merck, India | 231-442-4 | resublimed Granules |
| K12 –Kruss tensiometer | Kruss Scientific, Germany | K100 | |
| Laminar air flow cabnet | Thermo Scientific, China | 1300 Series A2 | |
| LCMS | Agilent Technologies, USA | 1260 Infinity II | |
| Luria Broth | HIMEDIA, India | M575-500G | Powder |
| Methanol | Merck, India | 107018 | |
| Ninhydrin | Titan Biotech Limited, India | 1608 | |
| p- anisaldehyde | Sigma, USA | 204-602-6 | |
| Petri plate | Tarsons, India | 460090-90 MM | Radiation sterilized polypropylene |
| Saponin | Merck, India | 232-462-6 | |
| Sodium chloride | Merck, India | 231-598-3 | |
| Test tubes | Borosil, India | 9800U06 | Glass tubes |
| TLC plates | Merck, India | 1055540007 | |
| Vortex | GeNei, India | 2006114318 | |
| Water Bath | Julabo, India | SW21C |
Odniesienia
- Desai, J. D., Banat, I. M. Microbial production of surfactants and their commercial potential. Microbiology and Molecular Biology Reviews. 61 (1), 47-64 (1997).
- Banat, I. M. Biosurfactants production and possible uses in microbial enhanced oil recovery and oil pollution remediation: a review. Bioresource Technology. 51 (1), 1-12 (1995).
- Singh, A., Van Hamme, J. D., Ward, O. P. Surfactants in microbiology and biotechnology: Part 2. Application aspects. Biotechnology Advances. 25 (1), 99-121 (2007).
- Shah, N., Nikam, R., Gaikwad, S., Sapre, V., Kaur, J. Biosurfactant: types, detection methods, importance and applications. Indian Journal of Microbiology Research. 3 (1), 5-10 (2016).
- McClements, D. J., Gumus, C. E. Natural emulsifiers-Biosurfactants, phospholipids, biopolymers, and colloidal particles: Molecular and physicochemical basis of functional performance. Advances in Colloid and Interface Science. 234, 3-26 (2016).
- Nguyen, T. T., Youssef, N. H., McInerney, M. J., Sabatini, D. A. Rhamnolipid biosurfactant mixtures for environmental remediation. Water Research. 42 (6-7), 1735-1743 (2008).
- Maier, R. M., Soberon-Chavez, G. Pseudomonas aeruginosa rhamnolipids: biosynthesis and potential applications. Applied Microbiology and Biotechnology. 54 (5), 625-633 (2000).
- Banat, I. M., Makkar, R. S., Cameotra, S. S. Potential commercial applications of microbial surfactants. Applied Microbiology and Biotechnology. 53 (5), 495-508 (2000).
- Mulugeta, K., Kamaraj, M., Tafesse, M., Aravind, J. A review on production, properties, and applications of microbial surfactants as a promising biomolecule for environmental applications. Strategies and Tools for Pollutant Mitigation: Avenues to a Cleaner Environment. , 3-28 (2021).
- Sharma, J., Sundar, D., Srivastava, P. Biosurfactants: Potential agents for controlling cellular communication, motility, and antagonism. Frontiers in Molecular Biosciences. 8, 727070 (2021).
- Vijayakumar, S., Saravanan, V. Biosurfactants-types, sources and applications. Research Journal of Microbiology. 10 (5), 181-192 (2015).
- Curiel-Maciel, N. F., et al. Characterization of enterobacter cloacae BAGM01 producing a thermostable and alkaline-tolerant rhamnolipid biosurfactant from the Gulf of Mexico. Marine Biotechnology. 23 (1), 106-126 (2021).
- Nikolova, C., Gutierrez, T. Biosurfactants and their applications in the oil and gas industry: current state of knowledge and future perspectives. Frontiers in Bioengineering and Biotechnology. 9, (2021).
- Rastogi, S., Tiwari, S., Ratna, S., Kumar, R. Utilization of agro-industrial waste for biosurfactant production under submerged fermentation and its synergistic application in biosorption of Pb2. Bioresource Technology Reports. 15, 100706 (2021).
- Zargar, A. N., Lymperatou, A., Skiadas, I., Kumar, M., Srivastava, P. Structural and functional characterization of a novel biosurfactant from Bacillus sp. IITD106. Journal of Hazardous Materials. 423, 127201 (2022).
- Adnan, M., et al. Functional and structural characterization of pediococcus pentosaceus-derived biosurfactant and its biomedical potential against bacterial adhesion, quorum sensing, and biofilm formation. Antibiotics. 10 (11), 1371 (2021).
- Du Nouy, P. L. A new apparatus for measuring surface tension. The Journal of General Physiology. 1 (5), 521-524 (1919).
- Akbari, S., Abdurahman, N. H., Yunus, R. M., Fayaz, F., Alara, O. R. Biosurfactants-a new frontier for social and environmental safety: a mini review. Biotechnology Research and Innovation. 2 (1), 81-90 (2018).
- Bicca, F. C., Fleck, L. C., Ayub, M. A. Z. Production of biosurfactant by hydrocarbon degrading Rhodococcus ruber and Rhodococcus erythropolis. Revista de Microbiologia. 30 (3), 231-236 (1999).
- Kuyukina, M. S., et al. Recovery of Rhodococcus biosurfactants using methyl tertiary-butyl ether extraction. Journal of Microbiological Methods. 46 (2), 149-156 (2001).
- Philp, J., et al. Alkanotrophic Rhodococcus ruber as a biosurfactant producer. Applied Microbiology and Biotechnology. 59 (2), 318-324 (2002).
- Mutalik, S. R., Vaidya, B. K., Joshi, R. M., Desai, K. M., Nene, S. N. Use of response surface optimization for the production of biosurfactant from Rhodococcus spp. MTCC 2574. Bioresource Technology. 99 (16), 7875-7880 (2008).
- Shavandi, M., Mohebali, G., Haddadi, A., Shakarami, H., Nuhi, A. Emulsification potential of a newly isolated biosurfactant-producing bacterium, Rhodococcus sp. strain TA6. Colloids and Surfaces B, Biointerfaces. 82 (2), 477-482 (2011).
- White, D., Hird, L., Ali, S. Production and characterization of a trehalolipid biosurfactant produced by the novel marine bacterium Rhodococcus sp., strain PML026. Journal of Applied Microbiology. 115 (3), 744-755 (2013).
- Najafi, A., et al. Interactive optimization of biosurfactant production by Paenibacillus alvei ARN63 isolated from an Iranian oil well. Colloids and Surfaces. B, Biointerfaces. 82 (1), 33-39 (2011).
- Bezza, F. A., Chirwa, E. M. N. Pyrene biodegradation enhancement potential of lipopeptide biosurfactant produced by Paenibacillus dendritiformis CN5 strain. Journal of Hazardous Materials. 321, 218-227 (2017).
- Jimoh, A. A., Lin, J. Biotechnological applications of Paenibacillus sp. D9 lipopeptide biosurfactant produced in low-cost substrates. Applied Biochemistry and Biotechnology. 191 (3), 921-941 (2020).
- Liang, T. -. W., et al. Exopolysaccharides and antimicrobial biosurfactants produced by Paenibacillus macerans TKU029. Applied Biochemistry and Biotechnology. 172 (2), 933-950 (2014).
- Mesbaiah, F. Z., et al. Preliminary characterization of biosurfactant produced by a PAH-degrading Paenibacillus sp. under thermophilic conditions. Environmental Science and Pollution Research. 23 (14), 14221-14230 (2016).
- Quinn, G. A., Maloy, A. P., McClean, S., Carney, B., Slater, J. W. Lipopeptide biosurfactants from Paenibacillus polymyxa inhibit single and mixed species biofilms. Biofouling. 28 (10), 1151-1166 (2012).
- Gudiña, E. J., et al. Novel bioemulsifier produced by a Paenibacillus strain isolated from crude oil. Microbial Cell Factories. 14 (1), 1-11 (2015).
- Pradhan, A. K., Pradhan, N., Sukla, L. B., Panda, P. K., Mishra, B. K. Inhibition of pathogenic bacterial biofilm by biosurfactant produced by Lysinibacillus fusiformis S9. Bioprocess and Biosystems Engineering. 37 (2), 139-149 (2014).
- Manchola, L., Dussán, J. Lysinibacillus sphaericus and Geobacillus sp biodegradation of petroleum hydrocarbons and biosurfactant production. Remediation Journal. 25 (1), 85-100 (2014).
- Bhardwaj, G., Cameotra, S. S., Chopra, H. K. Biosurfactant from Lysinibacillus chungkukjangi from rice bran oil sludge and potential applications. Journal of Surfactants and Detergents. 19 (5), 957-965 (2016).
- Gaur, V. K., et al. Rhamnolipid from a Lysinibacillus sphaericus strain IITR51 and its potential application for dissolution of hydrophobic pesticides. Bioresource Technology. 272, 19-25 (2019).
- Habib, S., et al. Production of lipopeptide biosurfactant by a hydrocarbon-degrading Antarctic Rhodococcus. International Journal of Molecular Sciences. 21 (17), 6138 (2020).
- Shao, P., Ma, H., Zhu, J., Qiu, Q. Impact of ionic strength on physicochemical stability of o/w emulsions stabilized by Ulva fasciata polysaccharide. Food Hydrocolloids. 69, 202-209 (2017).
- . Overview of DLVO theory Available from: https://archive-ouverte.unige.ch/unige:148595 (2014)
- Kazemzadeh, Y., Ismail, I., Rezvani, H., Sharifi, M., Riazi, M. Experimental investigation of stability of water in oil emulsions at reservoir conditions: Effect of ion type, ion concentration, and system pressure. Fuel. 243, 15-27 (2019).
- Chong, H., Li, Q. Microbial production of rhamnolipids: opportunities, challenges and strategies. Microbial Cell Factories. 16 (1), 1-12 (2017).
- Zeng, G., et al. Co-degradation with glucose of four surfactants, CTAB, Triton X-100, SDS and Rhamnolipid, in liquid culture media and compost matrix. Biodegradation. 18 (3), 303-310 (2007).
- Liu, G., et al. Advances in applications of rhamnolipids biosurfactant in environmental remediation: a review. Biotechnology and Bioengineering. 115 (4), 796-814 (2018).
- John, W. C., Ogbonna, I. O., Gberikon, G. M., Iheukwumere, C. C. Evaluation of biosurfactant production potential of Lysinibacillus fusiformis MK559526 isolated from automobile-mechanic-workshop soil. Brazilian Journal of Microbiology. 52 (2), 663-674 (2021).
- Naing, K. W., et al. Isolation and characterization of an antimicrobial lipopeptide produced by Paenibacillus ehimensis MA2012. Journal of Basic Microbiology. 55 (7), 857-868 (2015).
- Wittgens, A., et al. Novel insights into biosynthesis and uptake of rhamnolipids and their precursors. Applied Microbiology and Biotechnology. 101 (7), 2865-2878 (2017).
- Rahman, K., Rahman, T. J., McClean, S., Marchant, R., Banat, I. M. Rhamnolipid biosurfactant production by strains of Pseudomonas aeruginosa using low-cost raw materials. Biotechnology Progress. 18 (6), 1277-1281 (2002).
- Bahia, F. M., et al. Rhamnolipids production from sucrose by engineered Saccharomyces cerevisiae. Scientific Reports. 8 (1), 1-10 (2018).
- Kim, C. H., et al. Desorption and solubilization of anthracene by a rhamnolipid biosurfactant from Rhodococcus fascians. Water Environment Research. 91 (8), 739-747 (2019).
- Nalini, S., Parthasarathi, R. Optimization of rhamnolipid biosurfactant production from Serratia rubidaea SNAU02 under solid-state fermentation and its biocontrol efficacy against Fusarium wilt of eggplant. Annals of Agrarian Science. 16 (2), 108-115 (2018).
- Wang, Q., et al. Engineering bacteria for production of rhamnolipid as an agent for enhanced oil recovery. Biotechnology and Bioengineering. 98 (4), 842-853 (2007).
- Câmara, J., Sousa, M., Neto, E. B., Oliveira, M. Application of rhamnolipid biosurfactant produced by Pseudomonas aeruginosa in microbial-enhanced oil recovery (MEOR). Journal of Petroleum Exploration and Production Technology. 9 (3), 2333-2341 (2019).
- Amani, H., Mehrnia, M. R., Sarrafzadeh, M. H., Haghighi, M., Soudi, M. R. Scale up and application of biosurfactant from Bacillus subtilis in enhanced oil recovery. Applied Biochemistry and Biotechnology. 162 (2), 510-523 (2010).
- Gudiña, E. J., et al. Bioconversion of agro-industrial by-products in rhamnolipids toward applications in enhanced oil recovery and bioremediation. Bioresource Technology. 177, 87-93 (2015).
- Sun, G., Hu, J., Wang, Z., Li, X., Wang, W. Dynamic investigation of microbial activity in microbial enhanced oil recovery (MEOR). Petroleum Science and Technology. 36 (16), 1265-1271 (2018).
- Jha, S. S., Joshi, S. J., SJ, G. Lipopeptide production by Bacillus subtilis R1 and its possible applications. Brazilian Journal of Microbiology. 47 (4), 955-964 (2016).
- Darvishi, P., Ayatollahi, S., Mowla, D., Niazi, A. Biosurfactant production under extreme environmental conditions by an efficient microbial consortium, ERCPPI-2. Colloids and Surfaces. B, Biointerfaces. 84 (2), 292-300 (2011).
- Al-Wahaibi, Y., et al. Biosurfactant production by Bacillus subtilis B30 and its application in enhancing oil recovery. Colloids and Surfaces. B, Biointerfaces. 114, 324-333 (2014).
- Moutinho, L. F., Moura, F. R., Silvestre, R. C., Romão-Dumaresq, A. S. Microbial biosurfactants: A broad analysis of properties, applications, biosynthesis, and techno-economical assessment of rhamnolipid production. Biotechnology Progress. 37 (2), 3093 (2021).
- Youssef, N., Simpson, D. R., McInerney, M. J., Duncan, K. E. In-situ lipopeptide biosurfactant production by Bacillus strains correlates with improved oil recovery in two oil wells approaching their economic limit of production. International Biodeterioration & Biodegradation. 81, 127-132 (2013).
- Ruckenstein, E., Nagarajan, R. Critical micelle concentration and the transition point for micellar size distribution. The Journal of Physical Chemistry. 85 (20), 3010-3014 (1981).
- de Araujo, L. L., et al. Microbial enhanced oil recovery using a biosurfactant produced by Bacillus safensis isolated from mangrove microbiota-Part I biosurfactant characterization and oil displacement test. Journal of Petroleum Science and Engineering. 180, 950-957 (2019).
- Banat, I. M., De Rienzo, M. A. D., Quinn, G. A. Microbial biofilms: biosurfactants as antibiofilm agents. Applied Microbiology and Biotechnology. 98 (24), 9915-9929 (2014).
- Klosowska-Chomiczewska, I., Medrzycka, K., Karpenko, E. Biosurfactants-biodegradability, toxicity, efficiency in comparison with synthetic surfactants. Research and Application of New Technologies in Wastewater Treatment and Municipal Solid Waste Disposal in Ukraine, Sweden, and Poland. 17, 141-149 (2013).
- Fernandes, P. A. V., et al. Antimicrobial activity of surfactants produced by Bacillus subtilis R14 against multidrug-resistant bacteria. Brazilian Journal of Microbiology. 38 (4), 704-709 (2007).
- Santos, D. K. F., Rufino, R. D., Luna, J. M., Santos, V. A., Sarubbo, L. A. Biosurfactants: multifunctional biomolecules of the 21st century. International Journal of Molecular Sciences. 17 (3), 401 (2016).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone