Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
A Simple and Inexpensive Running Wheel Model for Progressive Resistance Training in Mice
W tym Artykule
Podsumowanie
This procedure describes a translatable progressive loaded running wheel resistance training model in mice. The primary advantage of this resistance training model is that it is entirely voluntary, thus reducing stress for the animals and the burden on the researcher.
Streszczenie
Previously developed rodent resistance-based exercise models, including synergistic ablation, electrical stimulation, weighted-ladder climbing, and most recently, weighted-sled pulling, are highly effective at providing a hypertrophic stimulus to induce skeletal muscle adaptations. While these models have proven invaluable for skeletal muscle research, they are either invasive or involuntary and labor-intensive. Fortunately, many rodent strains voluntarily run long distances when given access to a running wheel. Loaded wheel running (LWR) models in rodents are capable of inducing adaptations commonly observed with resistance training in humans, such as increased muscle mass and fiber hypertrophy, as well as stimulation of muscle protein synthesis. However, the addition of moderate wheel load either fails to deter mice from running great distances, which is more reflective of an endurance/resistance training model, or the mice discontinue running nearly entirely due to the method of load application. Therefore, a novel high-load wheel running model (HLWR) has been developed for mice where external resistance is applied and progressively increased, enabling mice to continue running with much higher loads than previously utilized. Preliminary results from this novel HLWR model suggest it provides sufficient stimulus to induce hypertrophic adaptations over the 9 week training protocol. Herein, the specific procedures to execute this simple yet inexpensive progressive resistance-based exercise training model in mice are described.
Wprowadzenie
Skeletal muscle mass comprises approximately 40% of body mass in adult humans; thus, maintaining skeletal muscle mass throughout life is critical. Skeletal muscle mass plays an integral role in energy metabolism, maintaining core body temperature, and glucose homeostasis1. The maintenance of skeletal muscle is a balance between protein synthesis and protein degradation, but many gaps still exist in the understanding of the intricate molecular mechanisms that drive these processes. To study the molecular mechanisms that regulate the maintenance and growth of muscle mass, human subjects' research models often employ resistance exercise-based interventions, since mechanical stimuli play an integral role in the regulation of skeletal muscle mass. While human subjects research has been successful, the time necessary to exhibit adaptations and ethical concerns regarding invasive procedures (i.e., muscle biopsies) limit the quantity of data that can be obtained. While the adaptations to resistance exercise are fairly ubiquitous across mammalian species, animal models provide the benefit of being able to precisely control the diet and exercise regimen while also allowing for the collection of whole tissues throughout the body, such as the brain, liver, heart, and skeletal muscle.
Many resistance training models have been developed for use in rodents: synergistic ablation2, electrical stimulation3,4, weighted ladder climbing5, weighted sled pulling6, and canvassed squatting7. It is evident that all of these models, if done correctly, can be effective models to induce skeletal muscle adaptations, such as hypertrophy. However, the downfalls of these models are that they are mostly involuntary, not part of normal rodent behavior, time-/labor-intensive, and invasive.
Fortunately, many mouse and rat strains voluntarily run long distances when given access to a running wheel. Moreover, free-running wheel (FWR) exercise models do not rely on extensive conditioning, positive/negative reinforcement, or anesthesia to force movement or muscle activity8,9. Running activity depends greatly on mouse strain, sex, age, and an individual basis. Lightfoot et al. compared the running activity of 15 different mouse strains and found that daily running distance ranges from 2.93 km to 7.93 km, with C57BL/6 mice running the farthest, regardless of sex10. FWR is commonly accepted as an excellent model for inducing endurance adaptations in skeletal and cardiac muscles11,12,13,14,15,16; however, utilizing wheel running in resistance training models is less commonly investigated.
As one could suspect, the hypertrophic effect of wheel running might be augmented by adding resistance to the running wheel, termed loaded wheel running (LWR), thus requiring greater efforts to run on the wheel to more closely mimic resistance training. Using varied methods of load application, previous studies have demonstrated that the LWR model utilizing rats and mice routinely displayed increases in limb muscle mass of 5%-30% in a matter of 6-8 weeks17,18,19,20,21. Furthermore, D'hulst et al. demonstrated that a single bout of LWR led to a 50% greater increase in activation of the protein synthesis signaling pathway compared to FWR22. Wheel resistance has been most commonly applied by a friction-based, constant loading method, whereby a magnetic brake or tension bolt is utilized to apply wheel resistance12,19,23,24. One caveat of the friction-based, constant load method is that when moderate to high resistance is applied, the animal cannot overcome the high resistance to initiate movement of the wheel, effectively ceasing training. Most importantly, many of the cage and wheel systems used for rodent running wheel models are quite costly and require specialized equipment.
Recently, Dungan et al. developed a progressive weighted-wheel-running (PoWeR) model, which applies a load to the wheel asymmetrically via external masses adhered to a single side of the wheel. The unbalanced wheel loading and variable resistance of the PoWeR model are thought to encourage continued running activity and promote shorter bursts of loaded wheel running in mice, more closely imitating the sets and repetitions performed with resistance training17. Despite the average running distance being 10-12 km per day, the PoWeR model yielded a 16% and 17% increase in plantaris muscle wet mass and fiber cross-sectional area (CSA), respectively. Despite many practical advantages, the PoWeR model of LWR does have some limitations. As recognized by the authors, the PoWeR model is a high-volume "hybrid" stimulus that is reflective of a blended endurance/resistance exercise model (i.e., concurrent training in humans), as opposed to a more strictly resistance exercise-based model, potentially introducing an interference effect and contributing to the less pronounced hypertrophy or different mechanisms by which hypertrophy is induced25. Ensuring that a concurrent training phenomenon does not occur in what is intended to be a resistance exercise training model is imperative. Therefore, the PoWeR model was modified to develop a LWR model that utilizes higher loads than previously used to more closely resemble a resistance training model. Herein, details are provided for a simple and inexpensive 9 week progressive resistance training LWR model in C57BL/6 mice.
Access restricted. Please log in or start a trial to view this content.
Protokół
This study was approved by Appalachian State University's Institutional Animal Care and Use Committee (#22-05).
1. Animals
- Procure C57BL/6 mice from the in-house mouse colony.
NOTE: Male mice 5-8 months of age at the start of the study were used. Daily running activity peaks and plateaus at around 9-10 weeks of age26. Previous studies have demonstrated that old mice (22-24 months) will also perform loaded wheel running27. - House the mice individually in a standard rodent cage with a wire lid and keep the cage in a controlled environment (20-24 °C with a 12:12 h light:dark cycle).
- Provide standard rodent chow and water ad libitum.
2. Running wheel apparatus
- Running wheel setup:
NOTE: Running wheels are assembled/set up similarly for all running protocols, except for adding 1 g or 2.5 g load magnets.- Glue a single 1 g sensor magnet to the outer middle circumference of the running wheel (Figure 1).
- Use this wheel with a single 1 g sensor magnet for only the first week of wheel acclimation.
- Loaded wheel running (LWR; identical loading protocol to PoWeR17): Follow steps 2.1.4-2.1.6.
- Week 2 for LWR requires 2 g of load (see Table 1) .
- Glue two 1 g magnets side-by-side onto the outer circumference of the wheel (Figure 2A).
NOTE: Here, it is helpful to use tape to hold the magnets in place until the glue firmly dries; otherwise, they may be attracted to the sensor magnet and become dislodged. - Apply additional load in weeks 3, 4, and 6 by placing another 1 g magnet on top of either of the magnets already present.
NOTE: No glue is necessary as the magnets firmly adhere to one another. For example, with 6 g of load in week 6, the magnets will each be stacked three-high (Figure 2B). - High loaded wheel running (HLWR): Follow steps 2.1.8-2.1.11.
NOTE: The HLWR protocol requires three sets of wheels. Assembling different sets of wheels allows the researcher to reuse wheel setups for other mice once the wheel is thoroughly cleaned and sanitized (numbers of each set should be determined by the researcher based on cohort/group size). - The first set of wheels (required for week 2 only) will have a single 2.5 g magnet; glue (refer to the NOTE below Step 2.1.5) one 2.5 g magnet onto the outer circumference of the wheel (Figure 3A).
- The second set of wheels (required for week 3 only) will have two 2.5 g magnets; glue (refer to the NOTE below Step 2.1.5) two 2.5 g magnets side-by-side onto the outer circumference of the wheel (Figure 3B).
- The third set of wheels (required for week 4 and beyond) will have three 2.5 g magnets side-by-side; glue (refer to the NOTE below Step 2.1.5) three 2.5 g magnets side-by-side onto the outer circumference of the wheel (Figure 3C).
- Apply additional load for weeks 6 and 8 by placing another 2.5 g magnet on top of either of the magnets already present (Figures 3D, E).

Figure 1: Basic running wheel with single 1 g sensor magnet glued to the middle outer circumference of the wheel. Please click here to view a larger version of this figure.

Figure 2: Loaded running wheel (LWR) with sensor magnet and 1 g loading magnets. (A) Example of 2 g of load, two 1 g magnets glued side-by-side to the outer edge of wheel; (B) example of 6 g of load, two 1 g magnets glued side-by-side to the outer edge of wheel with an additional 4 g of load applied. Please click here to view a larger version of this figure.
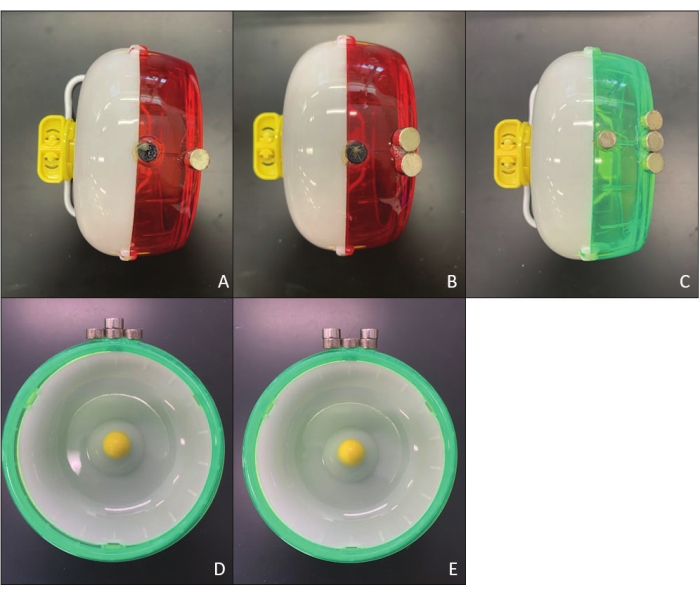
Figure 3: High loaded running wheel (HLWR) with sensor magnet and 2.5 g loading magnets. (A) example of 2.5 g of load, one 2.5 g magnet glued to the outer edge of wheel; (B) example of 5 g of load, two 2.5 g magnets glued side-by-side to the outer edge of wheel; (C) example of 7.5 g of load, three 2.5 g magnets glued side-by-side to the outer edge of wheel; (D) example of 10 g of load, three 2.5 g magnets glued side-by-side to the outer edge of wheel, with an additional 2.5 g of load applied; (E) example of 12.5 g of load, three 2.5 g magnets glued side-by-side to the outer edge of wheel, with an additional 5 g of load applied. Please click here to view a larger version of this figure.
3. Cage assembly
- Assemble running wheels using a cage equipped with a digital bike computer to monitor time exercising (h) and distance traveled (km). Average speed (km/h) is derived arithmetically.
- Ensure that a fresh battery is inserted into the bike computer prior to assembly.
- Set wheel size during initial bike computer programming (see manufacturer's instructions); calculate per revolution distance by measuring the outer circumference of the running wheel (e.g., 3,580 mm for the wheel type used herein).
- Place the bike computer sensor within a solid surface on the outside of the cage lid, directly above where the sensor magnet of the wheel is located. Ensure that all computer and sensor components are contained within a solid barrier outside the cage to prevent mice from chewing on components.
- Utilize the lid of an empty pipette tip box with a small rectangle cut out for the magnetic bike sensor to reside, and the main part of the box (with the tip rack grid removed) to hold the bike computer and wire (Figure 4A).
- Drill two holes through the corners of the solid surface to secure the magnetic bike sensor and running wheel stand in place on the outside of the cage (Figure 4A).
- Insert the running wheel base, upside down, through the gaps in the cage lid but on top of the solid surface described in step 3.2 (Figure 4B).
- Secure the wheel base and computer sensor to the top of the cage with hardware (Figure 4C, D).
- Ensure that the sensor magnet and computer sensor are spaced no more than 1 cm apart to allow for proper recording of wheel movement (most standard bike computer sensors are bi-directional and will record positive wheel movement in either direction of rotation).
- Attach the appropriate running wheel (as described above) to the wheel base from the inside of the cage lid, and securely place the lid onto the cage (Figure 4E, F).
- With the wheel hanging from the cage lid, ensure at least 2.5 cm of clearance from the cage floor. Place a minimal amount of bedding material in the cage to ensure that the wheel spins freely but does not become impeded by the buildup of bedding.
- During experimentation, record data from the bike computer at a consistent interval schedule to ensure accurate activity monitoring.
- Recognize that mice are a nocturnal species; therefore, most of their natural cage activity (including wheel running) will be performed during the dark hours of the light cycle.

Figure 4: Running wheel cage assembly. (A) Bike computer and magnetic sensor placed in solid surface/tray; (B) inverted wheel base placed on top of solid surface/tray and sensor (top view; note the two holes in sensor surface/tray for securing base to cage lid with hardware), (C) inverted wheel base with hardware assembled (bottom view); (D) inverted wheel base with hardware assembled (top view); (E) full cage assembly (top view); and (F) full cage assembly (side view). Please click here to view a larger version of this figure.
4. Exercise training loading protocols
- Individually house sedentary (SED) mice for 9 weeks in a cage containing a locked running wheel to prevent any running.
NOTE: Table 1 provides the loading schedule for the LWR (PoWeR) and HLWR protocols used in the experimental design. - Reduce load for the LWR and HLWR groups, if necessary, to ensure that mice continue to exercise for the entire 9 week protocol.
| Week | ||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||
| LWR (n = 4) | Load (g) | 0.0 | 2.0 | 3.0 | 4.0 | 5.0 | 5.0 | 6.0 | 6.0 | 6.0 |
| %BM | -- | 8% | 11% | 15% | 19% | 19% | 23% | 23% | 23% | |
| HLWR (n =7) | Load (g) | 0.0 | 2.5 | 5.0 | 7.5 | 7.5 | 10.0 | 10.0 | 12.5 | 12.5 |
| %BM | -- | 10% | 19% | 28% | 28% | 38% | 38% | 48% | 48% | |
Table 1. Loaded wheel running protocols
5. In situ muscle function testing, tissue harvesting, and tissue analysis
- Following the 9 week training intervention, anesthetize mice using inhaled isoflurane (4% induction; 2% maintenance) with supplemental oxygen, and ensure proper anesthetic plane monitoring throughout the procedure.
- Perform an in situ muscle function test on the gastrocnemius, plantaris, soleus (GPS) complex to test isometric muscular strength28. Establish a force-frequency curve by directly stimulating the sciatic nerve with 27 G electrode needles at 11 ascending frequencies between 1-300 Hz, with tetanic contractions occurring around 100-150 hz29.
- Immediately following the muscle function test, euthanize the mice via cervical dislocation and confirm euthanasia by removing the heart. Carefully excise the plantaris and soleus muscles and record the wet tissue mass.
- Coat each muscle sample in an embedding medium (OCT) and mount it on a cork. Freeze it in liquid nitrogen-cooled isopentane, and store it at -80 °C until further immunohistochemical (IHC) analysis is performed on sections of muscle tissue (10 µm thick).
- Analyze muscle fiber CSA using immunofluorescence for laminin. Measure fiber CSA using an automatic image quantification platform30.
6. Statistical analysis
- Express all data as mean ± SD.
- Perform statistical analyses using statistical analysis software with significance set at p ≤ 0.05.
- Compare wheel running and training volume data with repeated measures two-way ANOVA.
- Compare body mass, tissue mass, CSA, and muscle function with a one-way ANOVA. If significant F-ratios are found, compare within-group differences using Fisher LSD post hoc analyses.
- Calculate effect sizes, then interpret them as 0.01, 0.06, and 0.14 for small, medium, and large effect sizes, respectively.
Access restricted. Please log in or start a trial to view this content.
Wyniki
In this study, 24 C57BL/6 mice (6.3 ± 0.7 months at the start of this study) were randomly assigned to one of three treatment groups: sedentary (SED), loaded wheel running (LWR; same as PoWeR described by Dungan et al.17), or high LWR (HLWR), and then completed their respective 9 week protocol. After the acclimation week (week 1), there were no group or group x time differences in running distance or training volume (Figure 5).
Access restricted. Please log in or start a trial to view this content.
Dyskusje
Existing resistance exercise models in rodents have proven invaluable for skeletal muscle research; however, many of these models are invasive, involuntary, and/or time- and labor-intensive. LWR is an excellent model that not only induces similar muscular adaptations as those observed in other well-accepted resistance exercise training models, but also provides a chronic, low-stress exercise stimulus for the animal with minimal time/labor commitment by the researcher. Additionally, since LWR models require minimal direct...
Access restricted. Please log in or start a trial to view this content.
Ujawnienia
The authors have no conflicts of interest to disclose.
Podziękowania
We would like to thank the Graduate Student Government Association, Office of Student Research, and the Department of Health and Exercise Science at Appalachian State University for providing funding to support this project. Additionally, we would like to thank Monique Eckerd and Therin Williams-Frey for overseeing daily operations of the animal research facility.
Access restricted. Please log in or start a trial to view this content.
Materiały
| Name | Company | Catalog Number | Comments |
| 1 g disc neodymium magnets | Applied Magnets | ND018-6 | Used for all sensor magnets and 1 g increments of wheel loading |
| 2.5 g disc neodymium magnets | Applied Magnets | ND022 | Used for 2.5 g increments of wheel loading |
| 8-32 x 1" stainless steel screws | Amazon | https://www.amazon.com/gp/product/B07939RS23/ref=ppx_yo_dt_b_search_asin_title?ie=UTF8&psc=1 | |
| 8-32 Wing Nuts | Amazon | https://www.amazon.com/gp/product/B07YYWW2SB/ref=ppx_yo_dt_b_search_asin_title?ie=UTF8&th=1 | |
| 10 µL pipette tip box (empty) | Thermo Scientific | 2140 | We used empty ART Pipette tip boxes, but any similar sized boxes/trays would suffice |
| Extreme Liquid Glue | Loctite | ||
| Laminin primary antibody | Novus Biologicals | NB300-144AF647 | primary antibody conjugated with AF657; 1:200 in PBS containing 10% normal goat serum |
| Lithium 3 V battery | n/a | CR2032 | |
| M10 (3/16" x 1 1/4") stainless steel fender washers | Amazon | https://www.amazon.com/gp/product/B00OHUHEU8/ref=ppx_yo_dt_b_search_asin_title?ie=UTF8&th=1 | |
| MyoVision: Automated Image Quantification Platform | Wen et al. (2017) | v1.0 | https://www.uky.edu/chs/center-for-muscle-biology/myovision |
| Polycarbonate rodent cage (430 mm L x 290 mm W x 201 mm H), with narrow width stainless steel wired bar lid | Orchid Scientific | Polycarbonate Rat Cage Type II | https://orchidscientific.com/product/rat-cage/ - 1519974600758-c29bc1c5-6dfa |
| Sigma Sport 509 Bike Computer | Sigma Sport | Does not need to be this model in particular, but must have distance and time monitoring capabilities | |
| Silent Spinner Running Wheel (mini 11.4 cm) | Kaytee | SKU# 100079369 | https://www.kaytee.com/all-products/small-animal/silent-spinner-wheel |
Odniesienia
- Frontera, W. R., Ochala, J. Skeletal muscle: A brief review of structure and function. Calcified Tissue International. 96 (3), 183-195 (2015).
- Goldberg, A. L. Protein synthesis during work-induced growth of skeletal muscle. Journal of Cell Biology. 36 (3), 653-658 (1968).
- Baar, K., Esser, K. Phosphorylation of p70S6k correlates with increased skeletal muscle mass following resistance exercise. American Journal of Physiology-Cell Physiology. 276 (1), 120-127 (1999).
- Wong, T. S., Booth, F. W. Skeletal muscle enlargement with weight-lifting exercise by rats. Journal of Applied Physiology. 65 (2), 950-954 (1988).
- Hornberger Jr, T. A., Farrar, R. P. Physiological hypertrophy of the FHL muscle following 8 weeks of progressive resistance exercise in the rat. Canadian Journal of Applied Physiology. 29 (1), 16-31 (2004).
- Zhu, W. G., et al. Weight pulling: A novel mouse model of human progressive resistance exercise. Cells. 10 (9), 2459(2021).
- Tamaki, T., Uchiyama, S., Nakano, S. A weight-lifting exercise model for inducing hypertrophy in the hindlimb muscles of rats. Medicine and Science in Sports and Exercise. 24 (8), 881-886 (1992).
- De Bono, J. P., Adlam, D., Paterson, D. J., Channon, K. M. Novel quantitative phenotypes of exercise training in mouse models. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 290 (4), 926-934 (2006).
- Goh, J., Ladiges, W. Voluntary wheel running in mice. Current Protocols in Mouse Biology. 5 (4), 283-290 (2015).
- Lightfoot, J. T., Turner, M. J., Daves, M., Vordermark, A., Kleeberger, S. R. Genetic influence on daily wheel running activity level. Physiological Genomics. 19 (3), 270-276 (2004).
- Allen, D. L., et al. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. Journal of Applied Physiology. 90 (5), 1900-1908 (2001).
- Ishihara, A., et al. Effects of running exercise with increasing loads on tibialis anterior muscle fibres in mice. Experimental Physiology. 87 (2), 113-116 (2002).
- Kurosaka, M., et al. Effects of voluntary wheel running on satellite cells in the rat plantaris muscle. Journal of Sports Science and Medicine. 8 (1), 51-57 (2009).
- Lambert, M. I., Noakes, T. D. Spontaneous running increases VO2max and running performance in rats. Journal of Applied Physiology. 68 (1), 400-403 (1990).
- Rodnick, K. J., Reaven, G. M., Haskell, W. L., Sims, C. R., Mondon, C. E. Variations in running activity and enzymatic adaptations in voluntary running rats. Journal of Applied Physiology. 66 (3), 1250-1257 (1989).
- Sexton, W. L. Vascular adaptations in rat hindlimb skeletal muscle after voluntary running-wheel exercise. Journal of Applied Physiology. 79 (1), 287-296 (1995).
- Dungan, C. M., et al. Elevated myonuclear density during skeletal muscle hypertrophy in response to training is reversed during detraining. American Journal of Physiology-Cell Physiology. 316 (5), 649-654 (2019).
- Ishihara, A., Roy, R. R., Ohira, Y., Ibata, Y., Edgerton, V. R. Hypertrophy of rat plantaris muscle fibers after voluntary running with increasing loads. Journal of Applied Physiology. 84 (6), 2183-2189 (1998).
- Konhilas, J. P., et al. Loaded wheel running and muscle adaptation in the mouse. American Journal of Physiology-Heart and Circulatory Physiology. 289 (1), 455-465 (2005).
- Legerlotz, K., Elliott, B., Guillemin, B., Smith, H. K. Voluntary resistance running wheel activity pattern and skeletal muscle growth in rats: Wheel running activity pattern and muscle growth. Experimental Physiology. 93 (6), 754-762 (2008).
- Mobley, C. B., et al. Progressive resistance-loaded voluntary wheel running increases hypertrophy and differentially affects muscle protein synthesis, ribosome biogenesis, and proteolytic markers in rat muscle. Journal of Animal Physiology and Animal Nutrition. 102 (1), 317-329 (2018).
- D'Hulst, G., Palmer, A. S., Masschelein, E., Bar-Nur, O., De Bock, K. Voluntary resistance running as a model to induce mTOR activation in mouse skeletal muscle. Frontiers in Physiology. 10, 1271(2019).
- Soffe, Z., Radley-Crabb, H. G., McMahon, C., Grounds, M. D., Shavlakadze, T. Effects of loaded voluntary wheel exercise on performance and muscle hypertrophy in young and old male C57Bl/6J mice: Exercise and muscle hypertrophy in old mice. Scandinavian Journal of Medicine and Science in Sports. 26 (2), 172-188 (2016).
- White, Z., et al. Voluntary resistance wheel exercise from mid-life prevents sarcopenia and increases markers of mitochondrial function and autophagy in muscles of old male and female C57BL/6J mice. Skeletal Muscle. 6 (1), 45(2016).
- Murach, K. A., McCarthy, J. J., Peterson, C. A., Dungan, C. M. Making mice mighty: Recent advances in translational models of load-induced muscle hypertrophy. Journal of Applied Physiology. 129 (3), 516-521 (2020).
- Swallow, J. G., Garland, T., Carter, P. A., Zhan, W. -Z., Sieck, G. C. Effects of voluntary activity and genetic selection on aerobic capacity in house mice (Mus domesticus). Journal of Applied Physiology. 84 (1), 69-76 (1998).
- Murach, K. A., et al. Late-life exercise mitigates skeletal muscle epigenetic aging. Aging Cell. 21 (1), 13527(2022).
- Mackay, A. D., Marchant, E. D., Louw, M., Thomson, D. M., Hancock, C. R. Exercise, but not metformin prevents loss of muscle function due to doxorubicin in mice using an in situ method. International Journal of Molecular Sciences. 22 (17), 9163(2021).
- Godwin, J. S., Hodgman, C. F., Needle, A. R., Zwetsloot, K. A., Andrew, R. Whole-body heat shock accelerates recovery from impact- induced skeletal muscle damage in mice. Conditioning Medicine. 2 (4), 184-191 (2020).
- Wen, Y., et al. MyoVision: Software for automated high-content analysis of skeletal muscle immunohistochemistry. Journal of Applied Physiology. 124 (1), 40-51 (2018).
- Manzanares, G., Brito-da-Silva, G., Gandra, P. G. Voluntary wheel running: Patterns and physiological effects in mice. Brazilian Journal of Medical and Biological Research. 52 (1), 7830(2019).
- Bartling, B., et al. Sex-related differences in the wheel-running activity of mice decline with increasing age. Experimental Gerontology. 87, 139-147 (2017).
- Zwetsloot, K. A., Westerkamp, L. M., Holmes, B. F., Gavin, T. P. AMPK regulates basal skeletal muscle capillarization and VEGF expression, but is not necessary for the angiogenic response to exercise: AMPK and the skeletal muscle angiogenic response to exercise. The Journal of Physiology. 586 (24), 6021-6035 (2008).
Access restricted. Please log in or start a trial to view this content.
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone