Aby wyświetlić tę treść, wymagana jest subskrypcja JoVE. Zaloguj się lub rozpocznij bezpłatny okres próbny.
Method Article
Assessment of Mitochondrial Health in Cancer-Associated Fibroblasts Isolated from 3D Multicellular Lung Tumor Spheroids
* Wspomniani autorzy wnieśli do projektu równy wkład.
W tym Artykule
Podsumowanie
Multicellular 3D tumor spheroids were prepared with lung adenocarcinoma cells, fibroblasts, and monocytes, followed by the isolation of cancer-associated fibroblasts (CAFs) from these spheroids. Isolated CAFs were compared with normal fibroblasts to assess mitochondrial health by studying the mitochondrial transmembrane potential, reactive oxygen species, and enzymatic activities.
Streszczenie
Cancer-associated fibroblasts (CAFs) are among the most abundant stromal cells present in the tumor microenvironment, facilitating tumor growth and progression. Complexity within the tumor microenvironment, including tumor secretome, low-grade inflammation, hypoxia, and redox imbalance, fosters heterotypic interaction and allows the transformation of inactive resident fibroblasts to become active CAFs. CAFs are metabolically distinguished from normal fibroblasts (NFs) as they are more glycolytically active, produce higher levels of reactive oxygen species (ROS), and overexpress lactate exporter MCT-4, leading to the opening of the mitochondrial permeability transition pore (MPTP). Here a method has been described to analyze the mitochondrial health of activated CAFs isolated from the multicellular 3D tumor spheroids comprising of human lung adenocarcinoma cells (A549), human monocytes (THP-1), and human lung fibroblast cells (MRC5). Tumor spheroids were disintegrated at different time intervals and through magnetic-activated cell sorting, CAFs were isolated. The mitochondrial membrane potential of CAFs was assessed using JC-1 dye, ROS production by 2',7'-dichlorodihydrofluorescein diacetate (DCFDA) staining, and enzyme activity in the isolated CAFs. Analyzing the mitochondrial health of isolated CAFs provides a better understanding of the reverse Warburg effect and can also be applied to study the consequences of CAF mitochondrial changes, such as metabolic fluxes and the corresponding regulatory mechanisms on lung cancer heterogeneity. Thus, the present study advocates an understanding of tumor-stroma interactions on mitochondrial health. It would provide a platform to check mitochondrial-specific drug candidates for their efficacies against CAFs as potential therapeutics in the tumor microenvironment, thereby preventing CAF involvement in lung cancer progression.
Wprowadzenie
Solid tumors are composed of heterogeneous cell populations which are guided by the tumor microenvironment (TME), however, the origin of most of the cells has yet to be discovered. Mainly stromal and immune cells (fibroblasts, endothelial cells, monocytes, macrophages, dendritic cells, B cells, T cells, and their subsets) reflect the tumor heterogeneity in lung, breast, renal, and other solid cancers1,2,3. Understanding the origin of each subtype and their trans-differentiation potential is of utmost need for developing advanced therapies against these cancers. The analysis of this diverse cell population in human biopsies presents itself with several challenges because of the tumor type, site, stage, limitation of sample amount, and patient-specific variabilities4. Thus, an experimental model is needed, which is not only reliable but can also simulate the in vivo tumor condition, proving itself to be ideal for studying tumor-stroma crosstalk and its involvement in disease pathophysiology.
Three-dimensional (3D) multicellular tumor spheroid (MCTS) cultures are an advantageous in vitro model system of tumors due to their resemblance to natural counterparts. MCTS can better replicate aspects of solid tumors than 2D cell culture models, including their spatial architecture, physiological responses, the release of soluble mediators, gene expression patterns, and drug resistance mechanisms. Moreover, one main advantage of MCTS is that it can be used to study tumor heterogeneity and the tumor microenvironment (TME). The hanging-drop method is the most commonly employed tool for developing and analyzing MCTS5. In this method, the different cells with media are suspended in the form of droplets, which allows its growth in a coherent 3D aggregate fashion and is simple to access for examination. The technique is straightforward; it doesn't require many cells and eliminates the requirement of a specialized substrate like agarose for spheroid development6. An additional advantage of this method lies in the reproducibility of its technique. Furthermore, this method has also been used to co-culture mixed cell populations, such as endothelial cells and tumor cells, to simulate early tumor angiogenesis7.
In this study, multicellular 3D lung tumor spheroids were prepared with lung adenocarcinoma cells, fibroblasts, and monocytes using the hanging drop method which mimics the lung tumor microenvironment. Then the cancer-associated fibroblast (CAF) population was isolated to investigate mitochondrial health. The main idea behind developing these spheroids is to isolate the CAFs as the crosstalk among the cells in spheroids could transform the fibroblasts into a myo-fibroblast-like activated CAF state. Secondly, this study may also depict how aberrant ROS production and mitochondrial dysfunction drives the normal fibroblasts toward the more aggressive CAF phenotype. It was found that fibroblasts assembled within tumor spheroids gained myofibroblastic characteristics with increased ROS activity and the induction of metabolic gene expression. This protocol highlights the importance of the tumor microenvironment in activating CAF and could be an excellent model for in vitro generation and study of CAF phenotypic characteristics.
Protokół
1. Cell culture
- Culture human lung adenocarcinoma cell line A549, and human monocytic cell line THP-1 in RPMI1640 media supplemented with 10% FBS and 1% penicillin-streptomycin at 37 °C in a humidified chamber with 5% CO2.
- Culture MRC-5 human lung fibroblast cells in DMEM medium supplemented with 10% FBS and 1% penicillin-streptomycin solution at 37 °C in a humidified chamber with 5% CO2.
2. Preparation of multicellular tumor spheroids using A549 lung adenocarcinoma cell line, MRC5 fibroblasts, and THP-1 monocytes
NOTE: The multicellular tumorigenic and non-tumorigenic 3D spheroids were prepared using the hanging drop method in a 90 mm cell culture dish. A detailed description of these spheroids' development is given below. All cell culture reagents such as complete medium, PBS, and 0.25% trypsin-EDTA solution should be pre-warmed at 37 °C before use unless stated otherwise.
- Preparation of cell suspension
- Grow A549 and MRC5 adherent cells (5 x 106 cells each) in DMEM complete growth medium (Dulbecco's modified eagle medium [DMEM] + 10% fetal bovine serum [FBS] + 1% penicillin-streptomycin) in T25 flasks at 37 °C in a humidified chamber with 5% CO2.
- For THP-1 cells, grow the cell suspension (5 x 106 cells) in complete growth medium (RPMI1640 + 10% FBS + 1% penicillin-streptomycin) in T25 flasks. For better growth, place the T25 flasks at 37 °C in a humidified chamber with 5% CO2 in a standing position.
- After 3 days, at 80%-85% confluency, wash the A549 and MRC5 cells with calcium- and magnesium-free PBS (phosphate buffer saline) by adding 1 mL of PBS (25-30 °C) in each flask for 1 min and aspirating it.
- Harvest the A549 and MRC5 cells from the flask by incubating them with 500 µL of 0.25% trypsin-EDTA solution for 5 min at 37 °C in a humidified incubator with 5% CO2. Immediately after, add 4 mL of complete growth media to inactivate the trypsin.
- Collect the cell suspension from the T25 flask into a 15 mL tube and pellet it down at 125 x g for 5 min. Remove the supernatant and resuspend the cells in 5 mL of complete growth medium.
- Establishment of multicellular tumor spheroids
NOTE: All steps of the tumor spheroid formation should be performed inside the biosafety cabinet in order to maintain sterile conditions. The maximum volume of the cell suspension has been standardized as a drop of 25 µL to prepare a droplet such that it will not fall down while inverting the lids.- Count A549, MRC5, and THP-1 cell numbers using a cell counter. For each spheroid droplet (25 µL) maintain the following cell numbers: 5,000 A549 cells, 4,000 MRC5 cells, and 1,000 THP-1 cells, following the protocol described by Arora et al.7. Calculate the cell numbers accordingly for a 1 mL volume.
NOTE: Spheroids were initially prepared with three different cell counts per spheroid (i.e., 5000, 8000, and 10,000). Also, different cell ratios of tumor cells/fibroblasts/monocytes (1:1:1, 2:2:1, 4:2:1, 5:2:1, and 5:4:1) were checked. Finally, a successful 3D multicellular spheroid formation was seen with a ratio of 5:4:1 and was used for the study. Fibroblast concentration was increased based on its proportion in the tumor microenvironment, which further enhanced the rigidity of the tumor spheroids. The detailed procedure was reported in a recent publication by Arora et al.7. - Prepare the cell suspension at a ratio of 5 (A549, 2 x 105 cells/mL) to 4 (MRC5, 1.6 x 105 cells/mL) to 1 (THP-1, 5 x 104 cells/mL) and make up the volume to 1 mL with complete DMEM.
- Place a drop of 25 µL of cell suspension mixture onto the cover of a 90 mm culture dish (approximately 50 drops/90 mm dish). Fill the bottom of the 90 mm culture dish with 10 mL of sterile water.
- Carefully invert the lid over the water-filled hydration chamber and place the dish in a cell culture incubator for 3 days.
- Monitor the spheroids under the microscope at 10x magnification on day 4. To acquire images, switch on the power switch, place the 60 mm dish carefully on the stage, and select the magnification (10x). Adjust the lenses and examine the cells to analyze the cell aggregation and proliferation. Press the Freeze and Save buttons provided on the microscope to capture the image.
- Change the growth media on day 4 by carefully aspirating 20 µL of medium from each droplet and replacing it with a fresh complete growth medium.
- Count A549, MRC5, and THP-1 cell numbers using a cell counter. For each spheroid droplet (25 µL) maintain the following cell numbers: 5,000 A549 cells, 4,000 MRC5 cells, and 1,000 THP-1 cells, following the protocol described by Arora et al.7. Calculate the cell numbers accordingly for a 1 mL volume.
3. Live-dead analysis of tumor spheroids
- On day 7 and 10, carefully invert the 90 mm dish in the biosafety cabinet and use a 200 µL pipette to collect spheroids from each droplet. Collect five spheroids each in a 1.5 mL tube.
- Add 500 µL of 1x PBS to the 1.5 mL tube containing spheroids and centrifuge at 125 x g for 5 min. Discard the supernatant carefully and resuspend the spheroids in 200 µL of 1x PBS. Do not pipette rigorously to avoid spheroid disintegration.
- Pipette out the spheroids using a 200 µL pipette on a 60 mm dish for calcein-AM and propidium iodide staining.
- Put 5 µL of 1 µM calcein-AM solution and 5 µL of 2 mg/mL propidium iodide solution on the spheroids. Incubate for 10 min. After completion of the incubation period, wash the spheroids gently with 1x PBS twice.
- Place the 60 mm dish containing spheroids under the fluorescent inverted microscope, observe, and capture images at 10x magnification by selecting the fluorescence option in the microscope software and selecting the FITC (green fluorescence channel; excitation 490 nm, emission 515 nm) for calcein and Texas red channel (TXR; excitation 535 nm, emission 617 nm).
- For acquiring the image, switch on Ctr Adv, which is switch 1, followed by switching on the CPU. Wait for the software system to boot. When booted, place the 60 mm dish carefully on the stage and select the magnification (10x) and fluorescent channels (FITC, TXR). Adjust the lenses and scan for the image.
- To view the image in the system, select the option Live and view the image. Adjust the intensity of fluorescence for appropriate optimization. Save the image by clicking the Save button.
NOTE: At this stage, spheroids will be visible through the naked eye. Under the light microscope, spheroids will appear as round, rigid spheres at 10x magnification. Multiple spheroids can be collected at once using a 200 µL pipette.
4. Disintegration and cell suspension of tumor spheroids
- Collect 200 tumor spheroids each on day 7 and 10, using a 1 mL pipette in a 15 mL tube.
NOTE: Before collection, carefully check the shape and form of a single spheroid after transferring it onto a microscopic slide or on a 30 mm dish and observe under the microscope. - Pellet the spheroids by centrifugation at 125 x g for 5 min. Aspirate the supernatant carefully without disturbing the tumor spheroids.
- Carefully wash the spheroids with 200 µL of PBS, centrifuge at 125 x g for 5 min, and cautiously discard the supernatant.
- For the spheroid disintegration, add 400 µL of 0.25% trypsin-EDTA solution and keep at 37 °C for 10 min. Perform vigorous pipetting for complete disintegration of the spheroids.
- Neutralize trypsin by adding 1 mL of complete DMEM growth medium. Centrifuge at 125 x g for 5 min and discard the supernatant carefully.
- Resuspend the pellet in 1 mL of complete DMEM medium and count the total number of cells.
5. Cancer-associated fibroblast (CAF) isolation through microbeads
- For the isolation of CAFs from the tumor spheroids, resuspend 1 x 107 cells in 80 µL of cold magnetic activated cell sorting (MACS) buffer (PBS at pH 7.2 containing 0.5% bovine serum albumin [BSA] and 2 mM ethylenediamine tetraacetic acid [EDTA]).
- Incubate the cell suspension (containing 1 x 107 cells) with 20 µL of anti-fibroblast microbeads. Mix well by gently tapping the tube and incubate for 30 min at room temperature.
- Wash the cells with 1 mL of cold MACS buffer, centrifuge at 125 x g for 5 min, and aspirate the supernatant. Resuspend the cells in 500 µL of MACS buffer.
- For magnetic bead-based cell separation, prepare the MACS column by rinsing it with 3 mL of MACS buffer.
- Place the cell suspension into the column followed by the collection of flow-through containing the unlabeled cell population.
- Wash the column three times with 3 mL of MACS buffer. Remove the column from the separator and place it on the collection tube.
- Collect the anti-fibroblast microbead-labeled cells by adding 5 mL of MACS buffer and firmly pushing the plunger into the column.
- Centrifuge the labeled cells at 125 x g for 5 min. Proceed with the isolated fibroblasts for downstream applications.
6. Flow cytometry-based analysis of ACTA2 expression in isolated CAFs
- Count the number of isolated CAFs and use approximately 6 x 104 cells. Wash the cells once with PBS, centrifuge at 125 x g for 5 min, and aspirate the supernatant.
- Add 100 µL of cell permeabilization buffer (PBS + 0.5% BSA + 0.3% v/v Triton X-100) and incubate the cells at 4 °C for 30 min.
- Vortex the cells intermittently to maintain a single cell suspension. Centrifuge and resuspend the cells in 100 µL of cell permeabilization buffer.
- Add 2 µL of APC conjugated anti-human α-SMA antibody and incubate at 4 °C for 45 min. Following incubation, add 1 mL of permeabilization buffer and centrifuge at 125 x g for 5 min to remove excess antibody.
- Resuspend the pellet in 400 µL of permeabilization buffer for flow cytometric analysis. Acquire a total of 10,000 events of each sample in a flow cytometer. Select ACTA2 positive cells after distinguishing the populations of cells based on their forward and side scatter, selecting the singlet population followed by selecting the ACTA2 positive cells which appear as a single peak on the single parameter histogram.
7. JC-1 staining to determine mitochondrial membrane potential
- Count the number of isolated CAFs and use approximately 6 x 104 cells for 5,5,6,6'-tetrachloro-1,1',3,3' tetraethylbenzimi-dazoylcarbocyanine iodide (JC-1) staining using flow cytometry.
- Wash the cells thoroughly with PBS, centrifuge at 125 x g for 5 min, aspirate the supernatant, and add 100 µL of cell staining buffer.
- Add JC-1 dye at a working concentration of 2 µM and incubate at room temperature for 30 min. On termination of the incubation, wash the cells with PBS, centrifuge at 125 x g for 5 min, and resuspend in a final volume of 400 µL.
- Acquire a total of 20,000 events of each sample in a flow cytometer. Quantify the mitochondrial membrane potential by measuring the red-shifted JC-1 aggregates on the FL-2 channel and the green-shifted monomers on the FL-1 channel.
8. DCFDA staining to estimate cellular reactive oxygen species (ROS) levels
- Use the whole spheroids (50 numbers) as well as the isolated CAFs (6 x 104 cells) from the spheroids for 2',7'-dichlorodihydrofluorescein diacetate (DCFDA) staining.
- Wash the cells thoroughly with PBS, centrifuge at 125 x g for 5 min, aspirate the supernatant, and add 100 µL of cell staining buffer.
- Add DCFDA dye at a working concentration of 1 µM and incubate at room temperature for 30 min. Wash the cells twice with PBS, centrifuge at 125 x g for 5 min, and then resuspend in a final volume of 400 µL.
- Acquire a total of 20,000 events of each sample in a flow cytometer. Assess the ROS levels by measuring the fluorescence at day 7 and day 10 for the spheroids as well as the isolated CAFs.
9. RT-qPCR analysis of CAF markers and glycolytic genes
- Extract RNA from the sorted CAFs using a single-cell lysis kit following the manufacturer's protocol. Prepare cDNA from 100 ng of RNA using a cDNA synthesis kit following the manufacturer's guidelines.
- Perform RT-qPCR to analyze the relative CAF markers (ACTA28 and COL1A29) and glycolytic gene (GLUT110 and MCT411) expression. Perform melt curve analysis after the final extension to ensure the specificity of the products. Normalize the data using the expression of GAPDH as the reference gene. The primer sequences used for RT-qPCR are listed in Supplementary Table 1.
10. Extraction and quantification of the cellular protein from CAFs
NOTE: Perform all steps of protein extraction on ice to avoid protein degradation.
- Resuspend approximately 4 x 106 CAF cells in 100 µL of ice-cold RIPA lysis buffer (containing 30 mM HEPES, 150 mM NaCl, 1% NP-40, 0.1% SDS, and 0.5% sodium deoxycholate with 5 mM of Halt protease and phosphatase inhibitor cocktail, and 5 mM of EDTA at pH 7.4).
- Vortex rigorously for proper cell lysis. Sonicate the cell suspension at 20 Hz frequency, 20% amplitude for 15 s, and 3x pulse.
- After sonication, centrifuge the protein extract at 13,000 x g for 15 min at 4 °C. Transfer the supernatant to a pre-chilled 1.5 mL tube. Perform the BCA protein assay to quantify protein.
11. Spectrophotometric analysis of enzymatic activities in CAFs
NOTE: The following enzyme activities are analyzed in tumor spheroid derived CAFs.
- Measurement of succinate dehydrogenase activity
- To evaluate the activity of succinate dehydrogenase (SDH) in CAFs, prepare SHE buffer with 250 mM sucrose, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and 1 mM ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid(EGTA) and adjust the pH to 7.3. Add 0.4 mM phenazine methosulfate (PMS), 0.2 mM 2,6-dichloroindophenol (DCPIP), 50 mM MgCl2, 0.02% Triton X-100, and 1 mM cyanide in the SHE buffer and keep at 37 °C.
- Extract the cellular protein from the CAFs as described in step 10 (2 x 106 cells). In each well of a 96-well plate, incubate 100 µL of 0.1 mg cellular protein with 100 µL of SHE reaction mixture at 37 °C for 15 min.
- To start the enzymatic activity, add 10 mM succinate12. Calculate the enzyme activity in nM/min/mL by measuring the changes in absorbance at 600 nm.
- The conversion factor for DCIP is 0.0215 A/nM based on its molar extinction coefficient12. Calculate the relative activity of SDH by using the mentioned formula:
Relative activity (nM/min/mL/enzyme) =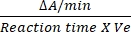 X
X  X V
X V
Where ΔA/min = rate of enzymatic reaction (Ainitial- Afinal)/(timefinal- A/mininitial), Ve = volume of sample, and V = volume of the reaction.
- Estimation of cytochrome c oxidase activity
- To evaluate the cytochrome c oxidase (COX) activity in CAFs, add 0.2 mg/mL of cellular protein in a KME reaction buffer solution containing KME buffer (125 mM KCl, 20 mM 3-(N-morpholino)propane sulfonic acid [MOPS], and 1 mM EGTA, pH 7.4), 0.02 % Triton X-100, and 5 mM sodium ascorbate.
- In a separate tube, mix 50 µM horse heart cytochrome c with 5 mM sodium ascorbate and incubate at 37 °C for 5 min13. After the incubation, add 20 µL of reduced cytochrome c to 500 µL of KME reaction buffer solution containing cellular protein.
- Calculate the enzyme activity in units/µL by measuring the changes in absorbance at 550 nm. The absorption of cytochrome c at 550 nm changes with its oxidation state. The difference in extinction coefficients (mM) between reduced and oxidized cytochrome c is 21.84 at 550 nm14. Calculate the amount of enzyme activity in the cellular lysate as:
Units/µL =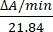
Where ΔA/min = A/minsample-A/minblank and 21.84 = ΔԑmM between oxidized cytochrome c and reduced cytochrome c at 550 nm
- Assessment of lactate dehydrogenase activity
- Perform a lactate dehydrogenase (LDH) assay using a lactate dehydrogenase cell assay kit following the manufacturer's instructions.
- Add LDH test reagent (10 µL) to 0.5 mg/mL of cellular proteins and incubate for 5 min at 37 °C. On termination of the incubation, measure the absorbance at 45 nm every 1 min for 8 min.
- Prepare the NADH standards for colorimetric detection using 0 (blank), 2.5, 5, 7.5, 10, and 12.5 nM/well of NADH followed by the addition of LDH test reagent to a final volume of 50 µL.
- Plot the differences in absorbance between Tinitial and Tfinal to the NADH standard curve to determine the amount of NADH generation. Assess the activity of LDH by using the mentioned formula:
LDH activity =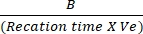 X sample dilution factor
X sample dilution factor
Where B = amount (nmole) of NADH generated between Tinitial and Tfinal, reaction time = Tfinal - Tinitial (min), and Ve = sample volume (mL) added to well.
Wyniki
Figure 1 shows the development of multicellular tumor spheroids using three different cell populations-A549 (lung adenocarcinoma), MRC-5 (fibroblasts), and THP-1 (monocytes)-by the hanging drop method as observed on day 7 and day 10 under the microscope. On day 7, spheroids were compact and rigid with a 260 ± 5.3 µm diameter, and on day 10, spheroids were 480 ± 7.5 µm in diameter (Figure 1A upper panel, Figur...
Dyskusje
The present study introduces the development of multicellular tumor spheroids comprising tumor cells, stromal cell population (i.e., fibroblasts), and immune cell population (i.e., monocytes) using a modified hanging drop method. Fibroblasts and monocytes/macrophages are among the most significant populations that constitute the tumor microenvironment (TME), and their presence is often linked with poor patient prognosis16. When present in the TME, fibroblasts transform, exhibiting a specific cance...
Ujawnienia
The authors have no conflicts of interest to disclose.
Podziękowania
This work was supported by the SERB-Women Excellence Award Project, India (SB/WEA-02/2017) and the SERB-Early Career Research Award Project, India (ECR/2017/000892) to DP. The authors, LA and SR acknowledge IIT Ropar and MHRD for their research fellowships. MK acknowledges ICMR for her research fellowship.
Materiały
| Name | Company | Catalog Number | Comments |
| Antibodies | |||
| APC anti-human α-SMA | R&D systems | Cat# IC1420A | |
| Anti-fibroblast microbeads | Miltenyi Biotec | Cat# 130-050-601 | |
| Cell lines | |||
| A549 lung adenocarcinoma cells | NCCS Pune | - | |
| MRC-5 fetal lung fibroblasts | ATCC | CCL-171 | |
| THP-1 Human monocytes | NCCS Pune | - | |
| Chemicals | |||
| BSA | Himedia | Cat# 9048-46-8 | |
| 2,6-dichloroindophenol (DCPIP) | SRL | Cat# 55287 | |
| Calcein-AM | Thermo Fisher Scientific | Cat# C3099 | |
| DAPI | Thermo Fisher Scientific | Cat# D1306 | |
| DCFDA | Sigma | Cat# D6883 | |
| DMEM | Gibco | Cat# 11995073 | |
| DPBS | Gibco | Cat# 14190-144 | |
| EDTA | Thermo fisher scientific | Cat# 17892 | |
| EGTA | SRL | Cat# 62858 | |
| EZcoun Lactate Dehydrogenase Cell Assay Kit | HiMedia | Cat# CCK036 | |
| FBS | Gibco | Cat# 10082147 | |
| Halt Protease and Phosphatase Inhibitor Cocktail (100X) | Thermo Fisher Scientific | Cat# 87786 | |
| HEPES | Thermo Fisher Scientific | Cat# 15630080 | |
| Horse heart Cytochrome c | SRL | Cat# 81551 | |
| Image-iT Red hypoxia reagent | Thermo Fisher Scientific | Cat# H10498 | |
| JC-1 Dye | Thermo Fisher Scientific | Cat# T3168 | |
| KCl | Merck | Cat# P9541 | |
| MgCl2 | Merck | Cat# M8266 | |
| MOPS | Thermo Fisher Scientific | Cat# 69824 | |
| Nacl | Sigma-Aldrich | Cat# S9888 | |
| NADH MB Grade | SRL | Cat# 54941 | |
| NP-40 | Thermo Fisher Scientific | Cat# 85124 | |
| Penicillin/Streptomycin | Gibco | Cat# 15140122 | |
| Phenazine methosulfate (PMS) | SRL | Cat# 55782 | |
| Propidium iodide | Thermo fisher scientific | Cat# P1304MP | |
| RPMI 1640 | Gibco | Cat# 11875093 | |
| Single Cell Lysis Kit | Thermo Fisher Scientific | Cat# 4458235 | |
| Sodium ascorbate | Merck | Cat# A7631 | |
| Sodium cyanide | Sigma | Cat# 205222 | |
| Sodium Deoxycholate | Thermo Fisher Scientific | Cat# 89904 | |
| Sodium dodecyl sulphate | Sigma-Aldrich | Cat# L3771 | |
| Sodium succinate hexahydrate | SRL | Cat# 36313 | |
| Sucrose | Sigma | Cat# S0389 | |
| SuperScript VILO cDNA synthesis kit | Thermo Fisher Scientific | Cat# 11754-050 | |
| Triton X-100 | Sigma | Cat# T8787 | |
| Trypsin 0.25% EDTA | Gibco | Cat# 25200072 | |
| Universal SYBR Green Supermix | BIO-RAD | Cat# 172-5124 | |
| Plasticware | |||
| MACS LS Columns | Miltenyi Biotec | Cat# 130-042-401 | |
| Equipment | |||
| Countess II FL Automated Cell Counter | Thermo Fisher Scientific | Cat# AMQAF1000 | |
| EVOS XL core imaging system | Thermo Fisher Scientific | Serial Number F0518-1727-0191 | |
| LAS X software | Leica Microsystems | ||
| Leica fluorescent inverted microscope | s | DMi8 automated S/N 424150) | |
| Midi MACS separator | Miltenyi Biotec | Cat# 130-042-302 |
Odniesienia
- Kim, N., et al. Single-cell RNA sequencing demonstrates the molecular and cellular reprogramming of metastatic lung adenocarcinoma. Nature Communications. 11 (1), 1-5 (2020).
- Davidson, S., et al. Single-cell RNA sequencing reveals a dynamic stromal niche that supports tumor growth. Cell Reports. 31 (7), 107628 (2020).
- Zhang, Y., et al. Single-cell analyses of renal cell cancers reveal insights into tumor microenvironment, cell of origin, and therapy response. Proceedings of the National Academy of Sciences. 118 (24), (2021).
- Bray, L. J., Hutmacher, D. W., Bock, N. Addressing patient specificity in the engineering of tumor models. Frontiers in Bioengineering and Biotechnology. 7, 217 (2019).
- Timmins, N. E., Nielsen, L. K. Generation of multicellular tumor spheroids by the hanging-drop method. Tissue Engineering. , 141-151 (2007).
- Dituri, F., et al. Complex tumor spheroid formation and one-step cancer-associated fibroblasts purification from hepatocellular carcinoma tissue promoted by inorganic surface topography. Nanomaterials. 11 (12), 3233 (2021).
- Arora, L., et al. Development of a multicellular 3D tumor model to study cellular heterogeneity and plasticity in NSCLC tumor microenvironment. Frontiers in Oncology. 12, 881207 (2022).
- Nurmik, M., Ullmann, P., Rodriguez, F., Haan, S., Letellier, E. In search of definitions: Cancer-associated fibroblasts and their markers. International Journal of Cancer. 146 (4), 895-905 (2020).
- Zhang, Y., et al. HIF-1α is necessary for activation and tumour-promotion effect of cancer-associated fibroblasts in lung cancer. Journal of Cellular and Molecular Medicine. 25 (12), 5457-5469 (2021).
- Bu, L., et al. Biological heterogeneity and versatility of cancer-associated fibroblasts in the tumor microenvironment. Oncogene. 38 (25), 4887-4901 (2019).
- Whitaker-Menezes, D., et al. Evidence for a stromal-epithelial "lactate shuttle" in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell cycle. 10 (11), 1772-1783 (2011).
- Mandujano-Tinoco, E. A., Gallardo-Pérez, J. C., Marín-Hernández, A., Moreno-Sánchez, R., Rodríguez-Enríquez, S. Anti-mitochondrial therapy in human breast cancer multi-cellular spheroids. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 1833 (3), 541-551 (2013).
- Bregman, A. A. . Laboratory Investigations in Cell and Molecular Biology. , (2002).
- Berry, E. A., Trumpower, B. L. Simultaneous determination of hemes a, b, and c from pyridine hemochrome spectra. Analytical Biochemistry. 161 (1), 1-15 (1987).
- Avagliano, A., et al. Metabolic reprogramming of cancer associated fibroblasts: the slavery of stromal fibroblasts. BioMed Research International. , (2018).
- Lorusso, G., Rüegg, C. The tumor microenvironment and its contribution to tumor evolution toward metastasis. Histochemistry and Cell Biology. 130 (6), 1091-1103 (2008).
- Liu, T., Zhou, L., Li, D., Andl, T., Zhang, Y. Cancer-associated fibroblasts build and secure the tumor microenvironment. Frontiers in Cell and Developmental Biology. 7, 60 (2019).
- Sebastian, A., et al. Single-cell transcriptomic analysis of tumor-derived fibroblasts and normal tissue-resident fibroblasts reveals fibroblast heterogeneity in breast cancer. Cancers. 12 (5), 1307 (2020).
- Elyada, E., et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discovery. 9 (8), 1102-1123 (2019).
- Ganguly, D., et al. Cancer-associated fibroblasts: Versatile players in the tumor microenvironment. Cancers. 12 (9), 2652 (2020).
- Harryvan, T. J., Verdegaal, E. M., Hardwick, J. C., Hawinkels, L. J., vander Burg, S. H. Targeting of the cancer-associated fibroblast-T-cell axis in solid malignancies. Journal of Clinical Medicine. 8 (11), 1989 (2019).
- Santi, A., Kugeratski, F. G., Zanivan, S. Cancer associated fibroblasts: the architects of stroma remodeling. Proteomics. 18 (5-6), 1700167 (2018).
Przedruki i uprawnienia
Zapytaj o uprawnienia na użycie tekstu lub obrazów z tego artykułu JoVE
Zapytaj o uprawnieniaPrzeglądaj więcej artyków
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. Wszelkie prawa zastrzeżone