A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Bioreactor Assembly for Continuous Culture of Complex Fecal Communities
In This Article
Summary
This protocol details the assembly of mini-bioreactor arrays to be utilized for continuous flow culture of complex fecal communities under anaerobic conditions. We also discuss the methods for assembly, inoculation, and sampling of the reactors for further analysis.
Abstract
The microbiota, especially bacteria, respond to various environmental exposures such as micro and macronutrients, pharmacological compounds, and inflammatory mediators, which dynamically alter community composition and microbial metabolic output. Understanding how physiological culture conditions affect microbial communities and diversity and their metabolic capacity will contribute important knowledge of their impact on health and diseases. Here, we present a protocol adapted from the template published by Auchtung et al. to create mini-bioreactor arrays that can cultivate complex fecal communities, define bacterial consortiums or single strains under precise conditions, including nutrient availability, temperature, flow rate, pH, and oxygen content. We describe the process for building the mini-bioreactor system, including adaptations to improve limitations in the published protocol. We also discuss the setup of the mini-bioreactor system under anaerobic conditions with the use of MEGA media (adapted from Han et al.), which is a rich media supporting the growth of diverse bacteria. We describe the inoculation of gut humanized mouse fecal samples into the mini-bioreactor system, followed by the establishment of complex community cultures within the mini-bioreactor system, which can be grown under continuous flow with aseptic sampling to monitor community composition. This system is adaptable to dietary changes and other cultural modifications. The techniques described here allow for the characterization of diverse, fastidious fecal communities under dynamic conditions or in response to perturbation in isolation from host-derived factors.
Introduction
The gut microbiota is a vast network of microorganisms, including bacteria, fungi, archaea, and viruses, which contain a huge genetic and metabolic repertoire that greatly outnumbers that of a human host1. The microbiome's rich metabolic capacity includes metabolites produced from bacterial processing of dietary nutrients, host metabolites chemically modified by bacteria, and metabolites synthesized uniquely by the microbiota2. Microbiota is implicated in nearly all host bioprocesses but also in disease states like inflammatory bowel disease, cancer, metabolic disorders, and even neurological disorders2,3. Dissecting the contribution of the microbiota from that of the host is essential to understanding the role of specific microorganisms or complex communities in health and disease. One approach that enables this dissection is the use of microbial bioreactors, which cultivate diverse microbial communities under dynamic conditions. There are many commercially available bioreactors that allow for the growth of fecal bacterial communities for almost any purpose, including large-scale bioproduct cultivation or precise control of specific nutritive factors for the study of metabolic processes. However, these systems are enormously costly and require extensive preparation, and do not enable diverse experimental conditions to be examined in parallel. For a simplified approach that is less costly, more adaptable, and allows for many parallel conditions, we present here the protocol for the setup and use of a mini-bioreactor array under continuous flow adapted from Auchtung et al.4.
The complete setup of the mini-bioreactor system is demonstrated in Figure 1A. This mini-bioreactor system uses arrays of 6 reactor wells, which are completely independent of one another and are set up in an anaerobic chamber under peristaltic flow to enable continuous media delivery and removal (Figure 1A). Each reactor array sits on a 60-position magnetic stir plate, with two 48-cassette peristaltic pumps holding 2-stop tubing for the source media and waste flow (Figure 1A). The anaerobic chamber is equipped with a CAM-12 anaerobic monitor for oxygen content, a catalyst box to remove oxygen with heating capacity, and a hydrogen sulfide removal column to absorb excess gas from bacterial metabolism. Each reactor well is equipped with a source media inlet, waste outlet, and sample port, which allows for inoculation or sampling of each individual reactor well (Figure 1B).
As described, the bioreactor system enables dynamic control over temperature, media consumption, and nutrient availability to support the cultivation and maintenance of fecal communities. Temperature is modulated by a catalyst box with heating capacity, enabling changes in temperature throughout the entire anaerobic chamber to be implemented quickly. Media consumption is regulated by peristaltic pump flow rates, which can be modified to change media turnover in the reactors. Media turnover can be customized to the community types being cultivated in reactors, for example, being adapted for slow-growing or very quickly growing target strains. Lastly, nutrient availability can be dynamically modified by adding components through the individual sample ports for each reactor well. Static conditions that can be modified at the outset of an experiment, but which do not have dynamic control in the system are media type, oxygen content, and number of simultaneous reactors. Investigators can choose to implement any source media they wish, and while one media is described here, endless other source media types have been successfully implemented. One can operate the reactors under aerobic or microaerophilic conditions (depending on the availability of a microaerophilic chamber). Lastly, as many or as few reactors as needed can be set up depending on the experimental needs of the investigator. While reactors are in sets of six within an array, each reactor operates independently of the others, and each can be linked to a different source if desired.
This modular bioreactor system can be completely set up for around $25,000 (not including the anaerobic chamber or chamber-specific components), with the only non-reusable part being the tubing which can be replaced for less than $100 per run of the system. The system is mostly automated but does require daily checking for media levels as media source bottles will need to be changed over when empty. Additionally, any sampling must be completed manually, and any dynamic conditional changes to the system will need to be initiated manually (for example, changing the temperature). From the original system protocol published by Auchtung et al., a few modifications were implemented to improve overall performance and utility4. Firstly, the material for 3D printing of the bioreactor array is modified to be ABS-like Translucent Clear Plastic to improve the structural integrity of the system through repeated rounds of autoclaving. Additionally, in place of the 1/8 inch (3.2 mm) OD PTFE tubing, E-LFL, 2.06 mm ID, 100 ft tubing is instead used due to the sturdier material, which is more resistant to collapse, which would cause blockages in flow in the system. Lastly, an additional fitting using 1/4 -28 mm thread to barbed male adaptors is used to reduce changes in tube slippage from media source bottles. From Han et al., the adapted MEGA media recipe provides an undefined, complex, highly rich media that enables the cultivation of fastidious anaerobic microorganisms5. The media is modified to utilize alternative preparation of the histidine-hematin component, as the original is no longer commercially available. Sodium hydroxide is also used to pH the media to 7.0 or any desired pH. Overall, this system is budget and user-friendly, optimized, and an excellent entry point to continuous flow culture systems.
In this protocol, we describe in detail the setup of this mini-bioreactor array system, including materials, sterilization of the system, and subsequent usage for cultivation of gut humanized murine fecal samples. The overall goal of this method is to build an adaptable and cost-effective bioreactor system that can be utilized for cultivating microbial communities under controlled conditions. A detailed schematic of the workflow for the assembly of the mini-bioreactor system is provided in Figure 2 and referenced at appropriate steps within the protocol. In our example, we inoculate two reactors with the same fecal sample from humanized gut mice and describe microbial community structure under a continuous flow of MEGA media.

Figure 1: Schematic diagram of mini-bioreactor array setup in the anaerobic chamber. (A) Full view of the completed mini-bioreactor array setup in the anaerobic chamber. The 6-well bioreactor array is situated on the 60-position magnetic stir plate. The source luer tee network is attached to the source media on the left-hand side through the two-hole bottle cap. Two peristaltic pumps hold the 2-stop tubing from the source and waste networks on the left and right, respectively. The waste luer tee network empties into the waste bottle on the right-hand side. Essential equipment for the operation of the anaerobic chamber, including the CAM-12 monitor, catalyst box with heating capacity, and hydrogen sulfide removal column, are arranged within the chamber around the array system. (B) Top view of the source, sample, and waste ports attached to one reactor well with media at the appropriate height in the reactor. Please click here to view a larger version of this figure.
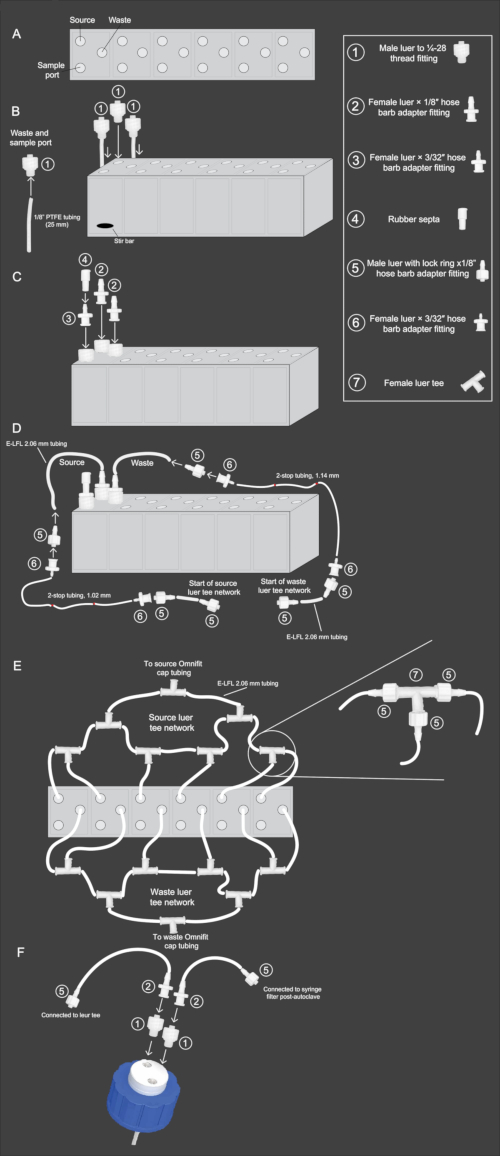
Figure 2: Schematic diagram of mini-bioreactor array assembly workflow. (A) Top-down view of the mini-bioreactor array, showing the orientation of the source, waste, and sample ports. (B) Side view of the mini-bioreactor array with view illustrating steps 2.3-2.4 of the protocol. The 1/8 inch PTFE tubing and fitting (1) are attached and inserted into the waste and sample ports. Fitting (1) without PTFE is inserted into the source port. (C) Side view of mini-bioreactor array illustrating steps 2.5-2.6 of the protocol. Fitting (2) is attached to the source and waste ports. Fitting (3) is attached to the sample port, and to that, fitting (4) is inserted on top. (D) Side view of mini-bioreactor array illustrating steps 2.9-2.11. E-LFL 2.06 mm tubing is connected to the source and waste ports, and the other end is connected to fittings (5) and then (6). 2-stop tubing of the appropriate diameter is then connected to fitting (6). The reverse connection is then repeated at the end of the 2-stop tubing, with fitting (6) connecting to the 2-stop tubing and fitting (5) connecting to that. Lastly, E-LFL 2.06 mm tubing will be connected to fitting (5) for connection to the luer tee network. (E) Top-down view of mini-bioreactor array displaying the source and waste luer tee network setup described in steps 2.12-2.15. E-LFL 2.06 mm tubing ending in fitting (5) is used to connect each reactor to several fittings (7), the last opening of which is connected to the cap on the source or waste bottle. (F) Side view of two-hole bottle cap assembly described in steps 2.16-2.17. One opening in the cap is connected to PTFE tubing that is placed inside the bottle. To that opening is inserted fitting (1), followed by fitting (2), and E-LFL 2.06 mm tubing connected to fitting (5). This fitting is attached to the source or waste luer network. To the opening not connected to PTFE tubing, fitting (1) is inserted, followed by fitting (2), and E-LFL 2.06 mm tubing is connected to another fitting (5). This end is capped with foil during autoclave sterilization for future attachment to a sterile 0.22 µM syringe filter. Please click here to view a larger version of this figure.
Protocol
Fecal samples used in this study were obtained from experiments approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Florida (UF) and performed at UF Animal Care Facilities (IACUC Protocol #IACUC202300000005). Briefly, mixed gender germ-free wild-type (GF WT; C57BL/6) mice (bred and maintained in isolators by UF Animal Care Services Germ-free Division) were transferred from breeding isolators and placed into the ISOcage P Bioexclusion system to allow for microbial manipulation. Equal colony-forming units (CFU) from human donor feces were pooled for gavage into mice. Two weeks post-inoculation, fecal samples were collected from these mice aseptically for storage and subsequent use in this protocol.
NOTE: Fecal sample collection and preparation described here are only intended as an example of a proper procedure, as depending on the type of samples collected (human, mouse, other animal) and the storage of these samples, this procedure can vary widely. Individuals should refer to the appropriate literature to design a protocol for fecal sample collection and preservation based on the needs of the individual researcher.
1. Typical preparation of fecal samples for -80 °C storage
- Select an anaerobic viability preservation media (here, we used Cary Blair). Aliquot 1 mL of preservation media into sterile, screw cap 2 mL tubes in a biosafety cabinet, and then transfer to the anaerobic chamber.
- Before receiving fecal samples, ensure all supplies (autoclaved spatulas, sterile saline, and screw-cap cryogenic vials containing preservation media) are pre-reduced for 24 h in the anaerobic chamber.
- Collect fecal samples aseptically from mice using sterile snap-cap tubes in a biosafety cabinet using autoclaved stool collection cups and forceps. Immediately upon collection, snap-freeze the tubes in liquid nitrogen.
- Immediately transfer samples to an anaerobic chamber along with a portable scale for weighing. Pre-weigh each tube. For mouse fecal samples, separate fecal pellets can be aliquoted per tube. If using human samples, use the sterile spatulas to stir each sample and divide it equally into cryogenic vials. After re-weighing each tube, add media at an estimated ratio of 1:10 feces media (mass: mass) to each tube for optimal preservation.
NOTE: Immediate anaerobic preservation of fecal samples is recommended following collection. Samples not immediately preserved may have reduced viability, compositional changes, etc., compared to freshly preserved samples6,7,8. Cary Blair preservation media maintains the viability of bacterial communities while inhibiting further growth and can be stored at -80 °C. For the estimated ratio of preservation media to feces, although the wet weight of the fecal sample is highly variable, this is the best estimate available. - Label, seal, remove vials from the anaerobic chamber, freeze flash with liquid nitrogen, and store at -80 °C for up to a year or more.
2. Building a 6-well reactor array
- Use the CAD file (Supplementary File 1) for 3D printing of the 6-well reactor. This file can be submitted to any 3D printing company for production. Use ABS-Like Translucent Clear plastic as the printing material to enable visualization through the reactors while also being resistant to repeated autoclaving cycles.
NOTE: We commissioned the 6-well reactor arrays to be made from ABs-like translucent Clear plastic by stereolithography. We recommend that the array be tilted (i.e., have the part 3D printed at a 30%-45% angle) in production, which reduces dimensional inaccuracies. This correction will result in visible layer lines running diagonally across the array, but this does not affect the reactors' performance. - Using a 25-28 mm tapping tool, introduce screw threads to each opening of the reactor array.
- Cut 12, 25 mm strips from the 1/8 inch PTFE tubing and insert one end of each segment of tubing into the screw thread opening of a male luer to 25-28 thread fitting. Securely screw the fittings into the designated waste and sample openings of the array (Figure 2B).
NOTE: A razor blade can be used to lightly shave the ends of the tubing into a cone-like configuration to make the tubing a better fit for the insertion. - Insert a micro stir bar into each reactor well of the reactor and then screw a male 1/4- 28 thread fitting into the source holes of each reactor well (Figure 2B).
- Screw female luer x 1/8 inch hose barb adapter fittings into the 1/4-28 thread fittings for the waste and source for each reactor well (Figure 2C).
- Screw female luer x 3/32 inch hose barb adapter fittings into the 1/4-28 thread fittings for the sample port. Place a rubber septum over the 3/32 inch hose barb adapters and fold the top over (Figure 2C).
- Cut two sets of E-LFL, 2.06 mm tubing in the following lengths for the waste and source lines: 14 cm, 15.25 cm, 16.5 cm, 17.75 cm, 19 cm, and 20.25 cm.
NOTE: Colored label tape wrapped around the midsection can be used to identify each length of tubing. - Attach the E-LFL 2.06 mm tubing to the female luer 1/8 inch hose barb adapter fittings for the source and waste connections (Figure 2D). Use hot water to make the tubing more malleable. This will allow the tubing to easily fit onto the adapters.
- Attach a male luer with a lock ring x 1/8 inch hose barb adapter fitting to the other end of the tubing. Screw a female luer x 1/16 inch hose barb adapter fitting into that (Figure 2D).
- Connect the appropriate size 2-stop tubing to the corresponding waste and source connections. The waste lines use 1.14 mm ID 2-stop tubing, while the source lines use 1.02 mm ID 2-stop tubing. On the other end of the 2-stop tubing, attach another female luer x 1/16 inch hose barb adapter fitting (Figure 2D).
- Cut 20, 5 cm pieces of E-LFL, 2.06 mm tubing, and insert male luer with lock ring x 1/8 inch hose barb adapter fittings to both ends of each tube. Attach one end of the 5 cm tubing with the male luer with lock ring x 1/8 inch hose barb adapter fitting to the unoccupied female luer x 1/16 inch hose barb adapter fitting at the end of each 2-stop tubing section. Repeat this process for the remaining 19 pieces of tubing (Figure 2D).
- Connect the E-LFL, 2.06 mm tubing for the source lines of reactor wells 1 and 2 using opposite ends of a female luer tee. Attach one of the 5 cm pieces of tubing with male luer with lock ring x 1/8 inch adapter fittings on each end to the middle outlet of the tee, leading to a second tee junction (Figure 2E).
- Connect the E-LFL, 2.06 mm source tubing for the third reactor, to the opposite side of the second tee. Another 5 cm tubing with a male luer with a lock ring x 1/8 inch adapter should be attached on one end, while the other end should have a female luer with a lock ring x 1/8 inch adapter attached. Follow the same pattern for reactors 4, 5, and 6 (Figure 2E).
- Cut a 25 cm piece of E-LFL, 2.06 mm tubing, attaching a male luer with lock ring x 1/8 inch hose barb adapter fitting to one end of the tubing, which will attach to the last remaining female luer tee junction, and on the other end attach a female luer x 1/8 inch hose barb adapter fitting. Cap this end with aluminum foil (Figure 2E).
NOTE: During the operation, it is essential to keep open ends sterile, so we recommend keeping them capped in foil during autoclaving and secured in place with tape. - Prepare the waste lines by repeating the same procedure as the source lines described in steps 2.12-2.14 (Figure 2E).
- To assemble a waste collection bottle, loosely secure a two-hole bottle cap onto a 2 L bottle. Insert male 1/4-28 thread fittings into both holes of the cap. Attach a female luer with 1/8 inch hose barb adapters to the male thread fitting that does not connect to the pre-attached PTFE tubing in the cap (Figure 2F).
- Next, cut a 5 cm piece of E-LFL 2.06 mm tubing and connect it to the hose barb adapter on the bottle. Attach a male luer with lock ring x 1/8 inch hose barb adapter to the other end of the 5 cm tubing and cover it with foil (this tubing serves as an air vent; after autoclaving, a 0.22 µm syringe filter will be added to prevent contamination; Figure 2F).
- Cover the entire cap with aluminum foil. Autoclave the reactors and waste bottle at 121 °C and a minimum of 15 psi for 45 min using the slow exhaust program typically set for liquids. Once the reactors have cooled, tighten the fittings and the aluminum foil seals and place them in the anaerobic chamber for 48 h before use.
3. MEGA media preparation
- Prepare all stock solutions as per Supplementary Table 1, Supplementary Table 2, Supplementary Table 3, Supplementary Table 4, Supplementary Table 5, Supplementary Table 6, Supplementary Table 7, Supplementary Table 8.
- In a 2 L glass bottle, add all dry ingredients from Supplementary Table 9, followed by 1640 mL of ultrapure water and then the rest of the Supplementary Table 10 liquid ingredients. Add a stir bar and dissolve completely over medium heat (90 °C) on a stir plate. The media will be pale blue to violet-colored and will not be fully clear but will not have any obvious un-dissolved lumps.
NOTE: Autoclaving glucose and other sugars in the presence of peptides can lead to modification and may lower available sugar in media. If desired, all sugar solutions can be sterilely filtered and added with the components of Supplementary Table 10, as described in step 3.7. - Once fully mixed, adjust the pH of the media to 7.0 by adding 10 N sodium hydroxide (NaOH), measuring exactly how much volume is added. After pH measurement, add 14.4 mL of water minus the volume of sodium hydroxide added to bring the volume to exactly 2 L.
- Prepare a two-hole bottle cap by inserting a male luer 1/4-28 mm thread fitting into both openings.
- In the hole of the cap not pre-attached to PTFE tubing, cut a 5 cm piece of C-flex tubing and connect it to the 1/8 inch hose barb of the female luer fitting. At the other end of the 5 cm tubing, insert another male luer with a lock ring and a 1/8 inch hose barb adapter, and cover with foil (this will also be connected to a 0.22 µM syringe filter).
- Cover the entire cap with aluminum foil and screw on top of the 2 L MEGA media bottle. Autoclave at 121 °C and a minimum of 15 psi for 45 min, using the slow exhaust program for liquids.
- Remove media from the autoclave and allow it to cool while stirring until lukewarm. Add the components in Supplementary Table 10 to the 2 L media bottle in a biosafety cabinet, seal the lid, and then move to a stir plate to homogenize. Once the media has cooled, tighten the fittings and the aluminum foil seals and place them in the anaerobic chamber for 48 h before use.
NOTE: We recommend running the source pump at 16 µL/min (0.94 mL/h) for a 16 h retention time. For this flow rate, a 6-well reactor uses approximately 0.135 L of media a day. We also recommend running the waste pump at 40 µL/min to prevent blockade forming in the waste tubing. Monitor the tubing and flow, noting that the waste flow can be increased as appropriate if blockages do occur. Both source and waste flow rates are modifiable, but investigators should carefully monitor their community composition and growth rates as these may also change with faster or slower flow. To run a 6 well reactor for one week, we recommend making at least 2L of media (it will consume a little more than 1 L).
4. Array setup in anaerobic chamber
NOTE: It is essential to clean the interior of the hood and the hood gloves with 3% hydrogen peroxide followed by triple deionized water rinse to ensure no surface contamination prior to assembly. If the connections are made quickly following the removal of the foil, and no direct contact is made with the interior of the fittings and the anaerobic chamber gloves, there is minimal chance of contamination
- Align the array over a magnetic stir plate in the anaerobic chamber. Ensure that the array is aligned correctly by setting the stir plate controller to maximum speed (1600 rpm) and observing the stir bars turning.
- Fit the 2-stop tubing for the source and waste in the clamps on the corresponding peristaltic pumps. If doing this less than 24 h before the intended use, lock the clamps in place. Otherwise, leave it loose until ready to use to reduce the wear on the tubing.
- Remove the foil cap on the female luer x 1/8 inch hose barb adapter fittings on the source and waste lines and screw into the male luer to 1/4 -28 threat fittings screwed into the caps (ensure that this opening is connected to the internal PTFE tubing).
- Remove the foil caps and attach a 0.22 µm syringe filter to the 5 cm vent line of the source bottle and to the vent line of the waste bottle.
- Begin the flow at a moderate rate (1 mL/min on the source pump and 2 mL/min on the waste pump) to begin filling the reactors. Ensure the waste line tube in each reactor well is lower than that of the source tube, which will prevent overfilling or the emptying of each well.
- As the reactors begin to fill, ensure that all reach the same volume and that media flows into the waste line. With the measurements described here, each reactor's working volume is 15 mL.
NOTE: If any individual reactors do not fill, tighten any loose fittings and inspect the source lines for issues, replacing them with prepared extras if necessary. If the issue persists, replace the source bottle if available. If the medium level rises above the interior waste line in the reactors, inspect that the PTFE tubing on the interior of the reactor waste port is still in place. - After each reactor has filled, adjust the pump settings to the 16 µL/min flow rate, and then turn off the pumps.
- Allow the medium to remain in the reactors and waste bottle(s) overnight before inoculation. This allows further anaerobic conditioning of the media (for a total of ~72 h) and enables the user to confirm the system's sterility.
- To confirm the sterility of the system prior to fecal sample inoculation, sample each reactor and the source media directly after initial filling (step 4.7) and again after step 4.8. Follow the procedure for aseptic sampling of the reactors described in step 6.
- Ensure that the optical density measurement of all samples is the same as that of the source media; if an increased optical density is observed, contamination is suspected, and all media and the system should be deconstructed, cleaned, and reassembled.
5. Fecal sample inoculation
- Place sterile wide bore pipette tips, sterile saline, 50 mL conical tubes, 25 mL sterile serological pipettes, 5 mL syringes, and 16-gauge needles into the anaerobic chamber to condition in the anaerobic environment for at least 24 h.
- Calculate the amount of fecal material required to inoculate the desired number of reactors. Each reactor well requires 3.8 mL of supernatant in a 25% m/v fecal slurry. For six reactor wells, a total of 22.8 mL of 25% m/v fecal slurry should provide enough supernatant for inoculation, which will require 5.7 g of solid fecal matter.
- Transfer the preserved fecal samples in cryogenic vials to the anaerobic chamber and allow them to thaw for 15 min.
- Use a wide-bore pipette tip to transfer fecal material from the cryogenic vials to a 50 mL conical tube, seal tightly, and centrifuge at 200 x g for 5 min to settle particulates.
- Transfer back into an anaerobic chamber and remove supernatant. Using an autoclaved laboratory spatula, transfer the fecal solids to a new 50 mL conical tube, achieving a total mass of 5.7 g. Add 17.1 mL of anaerobic sterile saline to the tube and seal tightly.
- Remove the conical tubes from the chamber, homogenize by vortex for 5 min, then centrifuge at 200 x g for 5 min to settle particulates.
- Sterilize the septa on top of each reactor with a 10% bleach laboratory wipe for at least 2 min while the samples are placed in the centrifuge.
- Return samples to the anaerobic chamber and, using sterile serological pipettes, transfer the supernatants to new 50 mL conical tubes.
- Load 3.8 mL of fecal slurry supernatant into six separate 5 mL syringes with 16 G 1.5 inch needles.
- Remove the wipe, insert the needle through the septum, and slowly inject the fecal slurry into the reactor.
- Allow the fecal communities to establish in each reactor for approximately 16-18 h before starting the flow to the reactor. Set the magnetic stir plate to maximum speed from the time the fecal slurry is injected (1600 rpm).
6. Aseptic collection of samples from reactor wells
- Puncture the wrapping on six sterile 1 mL syringes and six sterile 22G 3 inch needles and place them in the anaerobic chamber to degas for 24 h.
- Immediately before sampling, sterilize the septa on top of each reactor with a 10% bleach laboratory wipe for at least 2 min.
- Remove the bleach laboratory wipe and immediately attach a needle to a syringe and insert the needle into the center of the septa of the first reactor well.
- Pull up 1 mL of the sample, remove the needle from the septa, and transfer the sample from the syringe to a 1.7 mL labeled tube for downstream use. The rubber septa will reseal upon removal of the needle. Use a new needle and syringe for each reactor well to prevent cross-contamination.
- If the supernatant is desired, remove the tubes from the anaerobic chamber and centrifuge at 20,000 x g for 1 min to pellet bacterial cells.
- Transfer the supernatant into a separately labeled 1.7 mL tube and store at -80 °C.
7. Deconstructing and cleaning the mini-bioreactor system
- Disconnect the source bottle from the source luer tee and turn the flow rate up (1 mL/min on the source pump and 2 mL/min on the waste pump) to begin emptying the tubing. Allow the tubing to completely empty. Turn off the stir plate.
- Once the tubing is empty, disconnect the waste bottle from the waste luer tee. Then, place the reactor assembly into the anaerobic chamber airlock and remove it from the chamber.
- Disconnect all E-LFL 2.06 mm tubing and PTFE tubing segments and dispose of them. Disconnect all fittings and place them into a 10% bleach solution.
- Remove the mini-stir bars from each array well and place them in the bleach solution. Invert the reactor array to empty the media into a separate 10% bleach solution for disinfection. Then, submerge the empty array in a fresh bleach solution.
- Allow all fittings and the reactor array to soak in the 10% bleach solution for 30 min (extended soaking will cause discoloration).
- Rinse the fittings and reactor array with deionized water, then submerge overnight in enzymatic detergent solution according to manufacturer instructions.
- Triple rinse the fittings and reactor array with deionized water, then allow to completely air dry for at least 3 days. Once dry, the reactor array and fittings can be used for assembly once again.
Results
Fecal samples were collected from mice colonized with human fecal slurry 2 weeks post-inoculation and stored at -80 °C. The bioreactor system was set up with continuous MEGA media flow for two replicate reactor wells (Figure 3A). The fecal slurry was prepared from the mouse fecal pellets according to the protocol described in step 6, and both reactors were inoculated by sample port with the responder mouse fecal slurry. After overnight incubation of the ...
Discussion
The mini-bioreactor system described in this protocol enables the cultivation of independent fecal communities for parallel experimentation. This ability to study microbial communities in isolation of host factors is an essential approach to understanding the intrinsic capacity of microorganisms to adapt to their environment. This protocol can be easily adapted for the cultivation of defined bacterial consortia or even single isolate cultures if desired. The MEGA media described here is a rich, anaerobic broth designed f...
Disclosures
The authors have no conflicts of interest.
Acknowledgements
The authors are grateful to Josee Gauthier for the assistance with 16S rRNA gene sequencing. This research was supported, in part, by the UF Health Cancer Center Funds (C.J.) and UF Department of Medicine Gatorade Fund (C.J.). R.Z.G. was supported by UF Health Cancer Center funds. R.C.N. was supported by the National Institutes of Health TL1 Training Grant at the University of Florida (TL1TR001428, UL1TR001427), the National Cancer Institute of the National Institutes of Health Team-Based Interdisciplinary Cancer Research Training Program award T32CA257923 and the UF Health Cancer Center. Research reported in this publication was supported by the UF Health Cancer Center, supported in part by state appropriations provided in Fla. Stat. § 381.915 and the National Cancer Institute of the National Institutes of Health under Award Number P30CA247796. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the State of Florida. The funders had no role in study design, data collection, and analysis; decision to publish; or preparation of the manuscript.
Materials
| Name | Company | Catalog Number | Comments |
| 1 mL BD Slip Tip Syringe sterile, single use | Fisher Scientific | 309659 | |
| 1/4-28 mm thread to barbed male adaptor (3.2 mm), 5/pack | Cole-Parmer | 008NB32-KD5L | To build 1 6-well array, need 2 packs |
| 10M NaOH (Sodium Hydroxide) | Sigma | 72068-100ML | |
| 2.0ml Screw Cap Tube, NonKnurl, Skirted,Natural, E-Beam Sterile tube w/ attached cap | Fisher Scientific | 14-755-228 | |
| 2mag MIXdrive 60 Stirring Drive | 2mag | 40060 | 60-position magnetic stir plate (optional addition of heating capacity- cat# 40260) |
| 6-well reactor arrray, ABS-Like Translucent Clear plastic | Protolabs | Custom | See supplementary files for .stl file for 3D printing |
| Absolute Ethanol (200 Proof) | Fisher Scientific | BP2818 | |
| Acetic acid, glacial | Sigma | A6283 | |
| Adapter, nylon, male luer to 1/4-28 thread, 25/pack | Cole-Parmer | EW-45505-82 | To build 1 6-well array, need 6 (1 pack) |
| Aluminum foil | Fisher Scientific | 01-213-100 | |
| Anaerobic chamber | Coy Lab Products | Type B | |
| Bel-Art SP Scienceware Flea Micro Spinbar Magnetic Stirring Bars (1/pk) | Fisher Scientific | 22-261679 | To build 1 6-well array, need 6 |
| Biosafety cabinet class 2 | Nuaire | ||
| Butyric acid | Sigma | B103500 | |
| CaCl2 · 2H2O (Calcium Chloride Dihydrate) | Sigma | C7902 | |
| Clorox Healthcare Germicidal Wipes With Bleach, Unscented, 6" x 5", Pack Of 150 Wipes | Office Depot | 129202 | |
| D-(-)-Fructose | Sigma | F0127-100G | |
| D-(+)-Cellobiose | Sigma | C7252-100G | |
| D-(+)-Glucose | Sigma | G8270-100G | |
| D-(+)-Maltose monohydrate | Sigma | M5885-100G | |
| Drill America Plug Hand Tap DWTP1/4-28 | Home Depot | 305699489 | |
| Dulbecco's Phosphate Buffered Saline, 1X without Ca and Mg, Sterile | Genesee | 25-508 | |
| Female luer × 1/16″ hose barb adapter, Nylon, 25/pack | Cole-Parmer | EW-45502-00 | To build 1 6-well array, need 24 (1 pack) |
| Female luer × 1/8″ hose barb adapter, Nylon 25/pack | Cole-Parmer | EW-45502-04 | To build 1 6-well array, need 6 (1 pack) |
| Female luer × 3/32″ hose barb adapter, Nylon, 25/ pack | Cole-Parmer | EW-45502-02 | To build 1 6-well array, need 6 (1 pack) |
| Female luer tee, Nylon, 25/pack | Cole-Parmer | EW-45502-56 | To build 1 6-well array, need 10 (1 pack) |
| FeSO4 · 7H2O (Iron [II] Sulfate Heptahydrate) | Sigma | F8633 | |
| Hematin | Sigma | H3281 | |
| Histidine | Sigma | H7750 | |
| Isovaleric acid | Sigma | 129542 | |
| K2HPO4 dibasic (dipotassium hydrogen phosphate) | Sigma | P2222-1KG | |
| KH2PO4 monobasic (potassium dihydrogen phosphate) | Sigma | P0662-500G | |
| Large Orifice Pipet Tips | Fisher Scientific | 02-707-134 | |
| L-Cysteine hydrochloride | Sigma | C1276-10G | |
| Male luer with lock ring × 1/8″ hose barb adapter, Nylon, 25/pack | Cole-Parmer | EW-45505-04 | To build 1 6-well array, need 42 (2 packs) |
| Meat extract | Sigma | 70164-500G | |
| Med Vet International Exel Needle, 20G X 1", Hypodermic, 100/Box, 26417 | Fisher Scientific | 50-209-2532 | |
| Menadione (Vitamin K3) | Sigma | M5625 | |
| MgSO4 · 7H2O (Magnesium Sulfate Heptahydrate) | Sigma | M1880-500G | |
| Milli-Q water | |||
| NaCl (Sodium Chloride) | Sigma | S9888-500G | |
| NaHCO3 (Sodium Bicarbonate) | Sigma | S5761-500G | |
| Omnifi t Q-series two hole bottle cap | Cole-Parmer | 00945Q-2 | To build 1 6-well array, need 1 |
| PMP IPC-N L 24CHNL 8RLR 115V | MasterFlex | ISM939C-115V | 24-channel peristaltic pump, require 1 for source and 1 for waste |
| Precision Seal® rubber septa,white, 7 mm O.D. glass tubing (100/pk) | Millipore Sigma | Z553905-100EA | To build 1 6-well array, need 6 septa |
| Propionic acid | Sigma | P5561 | |
| Pump Tubing, 2-Stop, Tygon S3 E-Lab, 1.02 mm ID; 12/PK | VWR | MFLX96460-28 | To build 1 6-well array, need 6 (1 pack) |
| Pump Tubing, 2-Stop, Tygon S3 E-Lab, 1.14 mm ID; 12/PK | VWR | MFLX96460-30 | To build 1 6-well array, need 6 (1 pack) |
| Puritan Cary-Blair Medium, 5 ml | Fisher Scientific | 22-029-646 | |
| PYREX 2L Round Media Storage Bottles, with GL45 Screw Cap | Fisher Scientific | 06-414-1E | |
| Razor Blades | Genesee | 12-640 | |
| Resazurin, sodium salt | ACROS Organics from ThermoFisher | AC41890-0010 | |
| Soluble starch | Sigma | S9765-100G | |
| Stainless Steel Micro Spatulas, spoon like blade | Fisher Scientific | S50823 | |
| TBNG TYGON ELFL 2.06MMID 100' | VWR | MFLX06449-42 | To build 1 6-well array, need 205.5 cm |
| Trace Mineral Supplement | ATCC | MD-TMS | |
| Trypticase Peptone (BBL) | Fisher Scientific | B11921 | |
| Tubing, PTFE, 1/8″ (3.2 mm) OD × 1.5 mm ID, 10 M | Cole-Parmer | 008 T32-150-10 | To build 1 6-well array, need 300 mm |
| Tween 80 | Sigma | P1754 | |
| Vitamin Supplement | ATCC | MD-VS | |
| Yeast Extract (Bacto) | Fisher Scientific | DF0127-17-9 |
References
- Sender, R., Fuchs, S., Milo, R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol. 14 (8), e1002533 (2016).
- Postler, T. S., Ghosh, S. Understanding the holobiont: how microbial metabolites affect human health and shape the immune system. Cell Metab. 26 (1), 110-130 (2017).
- Rooks, M. G., Garrett, W. S. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 16 (6), 341-352 (2016).
- Auchtung, J. M., Robinson, C. D., Farrell, K., Britton, R. A. MiniBioReactor Arrays (MBRAs) as a tool for studying C. difficile physiology in the presence of a complex community. Methods Mol Biol. 1476, 235-258 (2016).
- Han, S., et al. A metabolomics pipeline for the mechanistic interrogation of the gut microbiome. Nature. 595 (7867), 415-420 (2021).
- Bilinski, J., et al. Fresh versus frozen stool for fecal microbiota transplantation-assessment by multimethod approach combining culturing, flow cytometry, and next-generation sequencing. Front Microbiol. 13, 872735 (2022).
- Li, X. M., et al. Effects of stool sample preservation methods on gut microbiota biodiversity: New original data and systematic review with meta-analysis. Microbiol Spectr. 11 (3), e0429722 (2023).
- Fouhy, F., et al. The effects of freezing on faecal microbiota as determined using MiSeq sequencing and culture-based investigations. PLoS One. 10 (3), e0119355 (2015).
- Sun, X., et al. Microbiota-derived metabolic factors reduce Campylobacteriosis in mice. Gastroenterology. 154 (6), 1751-1763.e2 (2018).
- He, Z., et al. Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut. 68 (2), 289-300 (2019).
- Newsome, R. C., et al. Interaction of bacterial genera associated with therapeutic response to immune checkpoint PD-1 blockade in a United States cohort. Genome Med. 14 (1), 35 (2022).
- Ziv, N., Brandt, N. J., Gresham, D. The use of chemostats in microbial systems biology. J Vis Exp. (80), e50168 (2013).
- Wille, J., Coenye, T. Biofilm dispersion: The key to biofilm eradication or opening Pandora's box. Biofilm. 2, 100027 (2020).
- Biagini, F., et al. Designs and methodologies to recreate in vitro human gut microbiota models. Bio-des Manuf. 6 (3), 298-318 (2023).
- Qi, Y., Yu, L., Tian, F., Zhao, J., Zhai, Q. In vitro models to study human gut-microbiota interactions: Applications, advances, and limitations. Microbiol Res. 270, 127336 (2023).
- Gościniak, A., Eder, P., Walkowiak, J., Cielecka-Piontek, J. Artificial gastrointestinal models for nutraceuticals research-achievements and challenges: A practical review. Nutrients. 14 (13), 2560 (2022).
- Marrero, D., et al. Gut-on-a-chip: Mimicking and monitoring the human intestine. Biosens Bioelectron. 181, 113156 (2021).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved