Seleção de Células Ativadas por Magnetismo (MACS): Isolamento de Linfócitos T Tímicos
Visão Geral
Fonte: Meunier Sylvain1,2,3, Perchet Thibaut1,2,3, Sophie Novault4, Rachel Golub1,2,3
1 Unidade de Linfopose, Departamento de Imunologia, Instituto Pasteur, Paris, França
2 INSERM U1223, Paris, França
3 Université Paris Diderot, Sorbonne Paris Cité, Cellule Pasteur, Paris, França
4 Flow Cytometry Platfrom, Cytometry and Biomarkers UtechS, Center for Translational Science, Pasteur Institute, Paris, França
A defesa contra patógenos depende da vigilância do sistema imunológico. Este sistema é complexo e compreende muitos tipos de células, cada uma com funções específicas. Essa composição complexa permite respostas imunes a uma grande diversidade de patógenos e lesões. A imunidade adaptativa permite respostas específicas contra patógenos específicos. A maioria das células responsáveis por esse tipo de imunidade são os linfócitos (células B e células T). Geralmente, as células B respondem a infecções extracelulares (como infecções bacterianas), e as células T respondem a infecções intracelulares (como infecções virais). Os diferentes tipos de células em populações de linfócitos podem ser caracterizados pela combinação de proteínas de superfície celular que expressam e/ou por um painel de citocinas secretadas.
A classificação magnética permite o enriquecimento de populações de células-alvo usando propriedades magnéticas e expressão de uma ou várias proteínas da superfície celular (1, 2). Esta técnica consiste em três passos. Primeiro, as células são incubadas com contas magnéticas que são acopladas a um ou vários anticorpos monoclonais específicos. Células que expressam proteínas superficiais que se ligam a esses anticorpos se prendem às contas magnéticas. Então, as populações de células-alvo são capturadas com um ímã. Para terminar, as células-alvo são eluidas do ímã. No final, são obtidos dois produtos de triagem, um contendo células não rotuladas e o segundo contendo as células-alvo, juntamente com as contas magnéticas. Colunas podem ser usadas para melhorar a eficiência da classificação magnética. Na coluna, um elemento não magnético alonga o caminho da célula através da coluna. Assim, o fluxo celular é desacelerado, facilitando a captura celular pelo ímã.
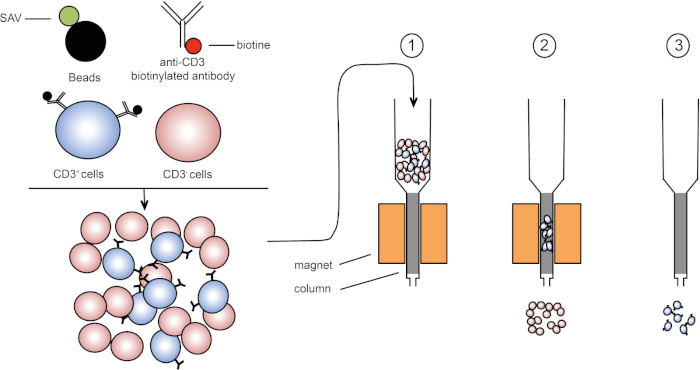
Figura 1: Representação esquemática da separação magnética. Leucócitos timimicos estão manchados com anticorpos biotinilados anti-CD3. Após a lavagem, streptavidin (SAV) acoplado contas especificamente fixam a biotina em anticorpos anti-CD3. (1) As células são transferidas em uma coluna. (2) O ímã não retém células não rotuladas, enquanto as células CD3 positivas permanecem na coluna. Finalmente, a coluna é separada do ímã e (3) células CD3 positivas são elucidadas em média. Clique aqui para ver uma versão maior desta figura.
Existem dois tipos de classificação magnética (3). Em classificação positiva, células de interesse são capturadas com as contas magnéticas. Na classificação negativa, as células indesejadas são removidas capturando com as contas magnéticas carregando os anticorpos apropriados. Esta técnica macs permite um bom enriquecimento de células-alvo e melhora a porcentagem de células recuperadas de 1-20% para 60-98% em um órgão. Após a triagem, é necessário verificar a pureza celular e a classificação por diferentes métodos (por exemplo, citometria de fluxo). A técnica MACS é ideal para enriquecer uma população-alvo para outros experimentos, como cultura celular ou análise de ciclo celular.
Neste exercício de laboratório, demonstramos como isolar leucócitos timiços e, posteriormente, enriquecer células timmicas CD3 positivas da mistura usando a técnica de classificação celular magnética.
Procedimento
1. Preparação
- Antes de começar, coloque luvas de laboratório e roupas de proteção apropriadas.
- Lave todas as ferramentas de dissecção, primeiro com um detergente e depois com 70% de etanol e depois seque-as com uma toalha de papel limpa.
- Prepare 200 mL da solução de sal balanceada da Hank (HBSS) contendo 2% de soro fetal de bezerro (FCS).
2. Dissecção
- Fixar um rato eutanizado em uma placa de dissecção na posição su
Resultados
Neste protocolo, as células CD3-positivas foram enriquecidas a partir de leucócitos timiêmicos usando classificação de células magnéticas (Figura 1). Antes do enriquecimento celular magnético, as células CD3-positivas representavam 53,6% do total das células timmicas (Figura 2, painéis superiores). Após o enriquecimento de células magnéticas, a porcentagem de células CD3 positivas aumentou para 95% (Figura 2, painéis inferiores). Assim, o MACS é uma técnica simples, ráp...
Aplicação e Resumo
A tecnologia de separação magnética é um método comum para classificar facilmente e rapidamente uma população celular alvo. Usando anticorpos específicos de células T e contas magnéticas enriquecemos a frequência de células T em nossa amostra. A taxa de pureza no final do experimento depende da porcentagem de células-alvo na suspensão celular inicial. As células obtidas após a classificação celular magnética podem ser usadas para vários propósitos, como transferência de células ou análise de ciclo...
Pular para...
Vídeos desta coleção:

Now Playing
Seleção de Células Ativadas por Magnetismo (MACS): Isolamento de Linfócitos T Tímicos
Immunology
23.1K Visualizações

Citometria de Fluxo e Separação de Células Ativadas por Fluorescência (FACS): Isolamento de Linfócitos B Esplênicos
Immunology
93.2K Visualizações

Ensaios ELISA: Indireto, Sanduíche e Competitivo
Immunology
239.4K Visualizações

Ensaio ELISPOT: Detecção de Esplenócitos Secretores de IFN-γ
Immunology
28.8K Visualizações

Imunohistoquímica e imunocitoquímica: imageamento de tecidos via microscopia de luz
Immunology
79.2K Visualizações

Geração de anticorpos: produzindo anticorpos monoclonais usando hibridomas
Immunology
43.7K Visualizações

Microscopia de imunofluorescência: Coloração por imunofluorescência de secções de tecido embebidos em parafina
Immunology
54.0K Visualizações

Microscopia confocal de fluorescência: uma técnica para determinar a localização de proteínas em fibroblastos de camundongos
Immunology
43.4K Visualizações

Técnicas Baseadas em Imunoprecipitação: Purificação de Proteínas Endógenas Usando Esferas de Agarose
Immunology
87.9K Visualizações

Análise do Ciclo Celular: Avaliação da Proliferação de Células T CD4 e CD8 Após Estimulação Usando Coloração CFSE e Citometria de Fluxo
Immunology
24.3K Visualizações

Transferência de células adotivas: introduzindo esplenócitos de camundongos doadores para um camundongo hospedeiro e avaliando o sucesso via FACS
Immunology
22.6K Visualizações

Ensaio para Morte Celular: Ensaio de Liberação de Cromo para Avaliar a Capacidade Citotóxica
Immunology
151.5K Visualizações
Copyright © 2025 MyJoVE Corporation. Todos os direitos reservados