자기 활성화 세포 분류 (MACS): 흉선 T 림프구 분리
Overview
출처: 뮤니에 실베인1,2,3,퍼셰 티보1,2,3,소피 노볼트4,레이첼 골럽1,2,3
1 림프포포에이시스, 면역학학과, 파스퇴르 연구소, 파리, 프랑스
2 INSERM U1223, 파리, 프랑스
3 유니버시테 파리 디드로, 소르본 파리 시테, 셀룰레 파스퇴르, 파리, 프랑스
4 흐름 세포측정플리트에서, 세포측정및 바이오마커 UtechS, 번역 과학 센터, 파스퇴르 연구소, 파리, 프랑스
병원체에 대한 방어는 면역 계통에 의한 감시에 달려 있습니다. 이 시스템은 복잡하고 많은 세포 유형, 특정 기능을 가진 각 세포 유형으로 구성됩니다. 이 복잡한 조성은 병원체와 상해의 큰 다양성에 면역 반응을 가능하게 합니다. 적응성 면역은 특정 병원체에 대한 특정 반응을 허용합니다. 면역의 이 모형을 책임지는 세포의 대다수는 림프구 (B 세포 및 T 세포)입니다. 일반적으로 B 세포는 세포 외 감염 (예 : 세균 감염)에 반응하고 T 세포는 세포 내 감염 (예 : 바이러스 감염)에 반응합니다. 림프구 집단에 있는 세포의 다른 모형은 그(것)들이 표현하는 세포 표면 단백질의 조합및/또는 분비한 사이토카인의 패널에 의해 특징지을 수 있습니다.
자기 선별은 자기 특성을 사용하여 표적 세포 집단의 농축을 허용하고 하나 또는 여러 세포 표면 단백질의 발현(1, 2). 이 기술은 세 단계로 구성됩니다. 첫째, 세포는 하나 또는 여러 개의 단일 클론 특이적 항체와 결합된 자기 구슬로 배양된다. 이러한 항체에 결합하는 표면 단백질을 표현하는 세포는 자기 구슬에 부착됩니다. 그런 다음 표적 세포 집단은 자석으로 캡처됩니다. 완료하려면 표적 세포가 자석에서 용출됩니다. 끝에, 2개의 선별 제품은 얻어지며, 하나는 라벨이 없는 세포를 포함하고 두 번째는 자기 비드와 결합된 표적 세포를 포함하는 것이다. 컬럼을 사용하여 자기 정렬의 효율성을 향상시킬 수 있습니다. 열에서 비자기 요소는 열을 통해 셀의 경로를 길게 합니다. 따라서 세포 흐름이 느려지고 자석에 의한 세포 캡처를 용이하게합니다.
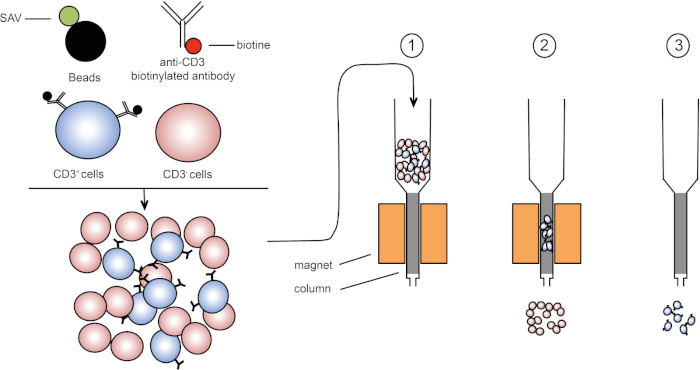
그림 1: 자기 분리의 회로도 표현. 흉백구세포는 항 CD3 바이오티니화 항체로 염색된다. 세척 후, 스트렙타비딘 (SAV) 결합 된 구슬은 특히 안티 CD3 항체에 비오틴을 고정합니다. (1) 셀은 열로 전송됩니다. (2) 자석은 표지되지 않은 세포를 유지하지 않으며 CD3 양성 셀은 열에 남아 있습니다. 마지막으로, 컬럼은 자석으로부터 분리되고 (3) CD3 양성 세포는 매체로 용출된다. 이 그림의 더 큰 버전을 보려면 여기를 클릭하십시오.
자기 선별 (3)의 두 가지 유형이 있습니다. 양색 선별에서 관심 있는 셀은 자기 구슬로 포획됩니다. 음의 선별에서 원치 않는 세포는 적절한 항체를 운반하는 자기 구슬로 캡처하여 제거됩니다. 이 MACS 기술은 표적으로 한 세포의 좋은 농축을 허용하고 기관에 있는 1-20%에서 60-98%로 복구된 세포의 비율을 향상합니다. 정렬 후, 셀 순도 및 다른 방법(예: 유동 세포측정)에 의해 정렬되는지 확인해야 합니다. MACS 기술은 세포 배양 또는 세포 주기 분석과 같은 그밖 실험을 위한 표적 인구를 풍부하게 하기 위하여 이상적입니다.
이 실험실 운동에서는, 우리는 자기 세포 분류 기술을 사용하여 혼합에서 흉선 백혈구를 분리하고 그 후에 백혈구를 풍부하게 하는 방법을 보여줍니다.
Procedure
1. 준비
- 시작하기 전에 실험실 장갑과 적절한 보호 복을 착용하십시오.
- 먼저 세제로 모든 해부 도구를 씻은 다음 70 % 에탄올로 씻은 다음 깨끗한 종이 타월로 말리십시오.
- 2% 태아 종아리 혈청(FCS)을 함유한 행크의 균형 잡힌 소금 용액(HBSS)의 200mL를 준비한다.
2. 해부
- supine 위치에 있는 해부 플레이트에 안락사 마우스를 고정합니다.
- 가위와 집게를 사용하여 흉부 구멍에 접근하기 위해 세로 복강경을 수행합니다.
- 심장을 제거하여 심장 위에 있는 흉선에 접근할 수 있습니다. 그런 다음, 두 개의 흰 엽으로 구성되고 심장 위의 가슴 구멍에 위치하는 흉통을 식별합니다.
- 포셉을 사용하여 흉선을 조심스럽게 분리하고 5mL의 HBSS 2 % FCS로 페트리 접시에 놓습니다.
Results
이 프로토콜에서, CD3 양성 세포는 자기 세포 분류를 사용하여 흉백세포로부터 농축되었다(도 1). 자기 세포 농축 CD3 양성 세포가 전체 흉구 세포의 53.6 %를 나타내기 전에 (그림 2, 상단 패널). 자기 세포 농축 후 CD3 양성 세포의 백분율은 95%로 증가했습니다(그림 2, 하단 패널). 따라서 MACS는 세포 현탁액 혼합물로부터 원하는 세포 집단을 풍부하게 하는 간단하고 빠르며 효율?...
Application and Summary
자기 분리 기술은 표적 세포 집단을 쉽고 빠르게 정렬하는 일반적인 방법입니다. T 세포를 사용하여 특정 항체와 자기 구슬우리는 우리의 견본에 있는 T 세포 주파수를 풍부하게 했습니다. 실험의 끝에 있는 순도율은 초기 세포 현탁액에 있는 표적 세포의 백분율의 달려 있습니다. 자기 세포 분류 후 얻은 세포는 세포 전달 또는 세포 주기 분석과 같은 다양한 목적을 위해 사용될 수 있다. 유동 세?...
Tags
건너뛰기...
이 컬렉션의 비디오:

Now Playing
자기 활성화 세포 분류 (MACS): 흉선 T 림프구 분리
Immunology
23.1K Views

유세포 분석 및 형광 활성화 세포 분류 (FACS): 비장 B 림프구의 분리
Immunology
93.2K Views

ELISA 분석: 간접, 샌드위치 및 길항
Immunology
239.4K Views

ELISPOT 분석: IFN-γ 분비 비장세포 검출
Immunology
28.8K Views

면역 조직 화학 및 면역 세포 화학: 광학 현미경을 통한 조직 이미징
Immunology
79.2K Views

항체 생성: 융합 세포를 사용한 단일 클론 항체 생성
Immunology
43.7K Views

면역 형광 현미경 검사법: 파라핀이 내장 된 조직 절편의 면역 형광 염색
Immunology
54.0K Views

공 초점 형광 현미경: 쥐 섬유 아세포에서 단백질의 국소화를 결정하는 기술
Immunology
43.4K Views

면역침전반응 기반 기술: 아가로스 비즈를 사용한 내인성 단백질의 정제
Immunology
87.9K Views

세포주기 분석: 세포주기 CFSE 염색 및 유세포 분석을 사용한 자극 후 CD4 및 CD8 T 세포 증식 평가
Immunology
24.3K Views

입양 세포 전송: 기증자 쥐 비장 세포를 숙주 쥐에 도입 후 FACS를 통한 성공 평가
Immunology
22.6K Views

세포 사멸 분석: 세포 독성 능력의 크롬 방출 분석
Immunology
151.5K Views
Copyright © 2025 MyJoVE Corporation. 판권 소유