Conjugação: um método para transferir a resistência à ampicilina da E. coli doadora para a receptora
Visão Geral
Fonte: Alexander S. Gold1, Tonya M. Colpitts1
1 Departamento de Microbiologia, Escola de Medicina da Universidade de Boston, Laboratórios Nacionais de Doenças de Infecções Emergentes, Boston, MA
Descoberta pela primeira vez por Lederberg e Tatum em 1946, a conjugação é uma forma de transferência genética horizontal entre bactérias que depende do contato físico direto entre duas células bacterianas (1). Ao contrário de outras formas de transferência genética, como transformação ou transdução, a conjugação é um processo natural em que o DNA é secretado de uma célula doadora para uma célula receptora de forma unidirecional. Essa direcionalidade e a capacidade desse processo de aumentar a diversidade genética das bactérias deram à conjugação a reputação como uma forma de "acasalamento" bacteriano, que acredita-se ter contribuído muito para o recente aumento de bactérias resistentes a antibióticos (2, 3). Ao utilizar pressões seletivas, por exemplo, o uso de antibióticos, a conjugação tem sido manipulada para uso em ambiente de laboratório, tornando-se uma ferramenta poderosa para a transferência horizontal de genes entre bactérias e, em alguns casos, de bactérias para leveduras, plantas e células animais (4). Além das aplicações em laboratório, a transferência de genes bacterium-eucayote por conjugação é uma avenida emocionante de transferência de DNA com uma infinidade de possíveis aplicações biotécnicas e implicações naturais (5).
Acredita-se que a conjugação funcione por um "mecanismo de duas etapas" (6). Primeiro, antes que qualquer DNA possa ser transferido, a célula doadora deve fazer contato direto entre células com o receptor. Esse processo tem sido caracterizado melhor em bactérias gram-negativas, sendo a mais estudada Escherichia coli. O contato celular-célula é estabelecido pela presença de uma complexa rede de filamentos extracelulares no doador conhecido como pilus sexual, um elemento conjugado codificado pelo gene transferível conhecido como fator F (fertilidade) (7, 8). Além de estabelecer contato entre doador e receptor, diversas proteínas são transportadas através do pilus sexual para o citoplasma receptor, formando um conduíte tipo IV (T4SS) entre as duas células, estrutura necessária para a segunda etapa de conjugação, transferência de DNA (6). Combinando essa função do pilus sexual com a replicação do DNA, a célula doadora é capaz de transferir DNA na forma de um elemento transposável, como um plasmídeo ou transposon, para o receptor por um modelo de "atirar e bombear" (6). Neste caso, o "tiro" é o transporte da proteína piloto, com DNA vinculado, pelo T4SS para a célula receptora, e o "bombeamento" é o transporte ativo de DNA para o receptor, um processo dependente do T4SS e catalisado por proteínas de acoplamento (6). O maquinário utilizado neste processo é composto por uma origem da sequência de transferência(oriT),que deve ser fornecida pelo DNA em genes cis e trans, que codificam um complexo de relaxase, formação de par de mate e proteína de acoplamento tipo IV, e pode estar presente em cis ou trans (9). Esta relaxase corta o local de nic dentro da sequência oriT e se prende covalentemente ao final da cadeia transferida para produzir o relaxosome, um complexo de relaxamento de DNA de uma única cadeia com outras proteínas auxiliares (9). Uma vez formado, o relaxosome conecta-se ao complexo de formação de par de acasalamento, através da proteína de acoplamento tipo IV, que permite a transferência do complexo ssDNA-relaxase para células receptoras pelo T4SS (10). Uma vez no citoplasma do receptor, o DNA pode se integrar ao genoma receptor ou existir separadamente na forma de um plasmídeo, qualquer um dos quais permite a expressão de seus genes.
Neste experimento, a cepa de doadores de conjugação amplamente utilizada E. coli WM3064 foi usada para transferir a codificação genética para resistência à ampicilina à cepa receptora E. coli J53. Enquanto ambas as cepas das bactérias gram-negativas eram resistentes à tetraciclina, apenas a cepa doadora WM3064 tinha o gene para resistência à ampicilina, codificada no vetor pWD2-oriT, e era auxotrófica ao ácido diaminopimelic (DAP) (11-13). Este experimento consistiu em duas etapas principais, a preparação de cepas de doadores e receptores, seguidas pela transferência do gene de resistência à ampicilina de doador para receptor por conjugação (Figura 1).
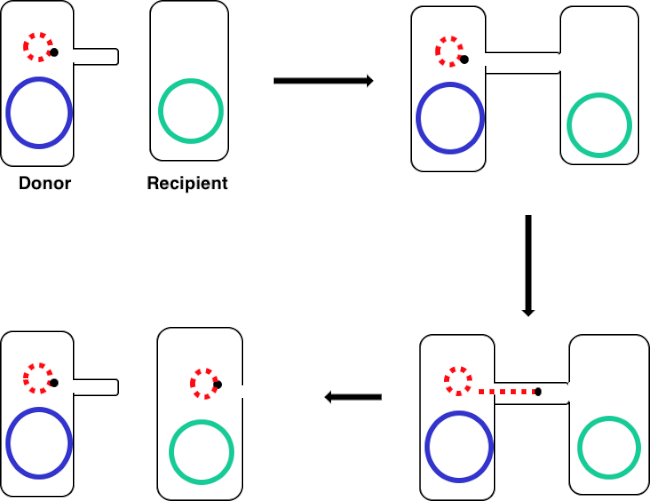
Figura 1: Esquema de conjugação. Este esquema mostra a transferência bem sucedida de um plasmídeo, apenas um exemplo de um elemento de DNA transposável, de uma célula doadora para uma célula receptora usando conjugação. Após o contato com a célula receptora pela célula doadora através do pilus sexual, o plasmid replica-se pela replicação do círculo de rolamento, move-se através do complexo multiproteína que une as duas células, e forma um novo plasmídeo de comprimento completo na célula receptora.
Ao incubar uma mistura de células doadoras e receptoras, em seguida, sucessivamente emplacando essas células na presença de tetraciclina e DAP, isso permitiu a transferência bem sucedida do gene de resistência à ampicilina. Sucessivamente, as células de revestimento cultivadas a partir dessa mistura na presença de tetraciclina e ampicilina, removeram todas as células doadoras devido à falta de DAP e de quaisquer células receptoras que possam não ter adquirido o gene de resistência à ampicilina, produzindo bactérias estritamente receptoras da cepa J53 que adquiriram resistência à ampicilina (Figura 2). Uma vez realizada, a transferência bem sucedida do gene de resistência à ampicilina foi confirmada pelo PCR. Desde que a conjugação foi bem sucedida, a cepa J53 de E. coli continha pWD2-oriT e era resistente à ampicilina, e a codificação genética para essa resistência é detectável pelo PCR. No entanto, se não tivesse havido nenhuma detecção do gene de resistência à ampicilina e a ampicilina ainda funcionaria como um antibiótico eficaz contra a cepa J53.
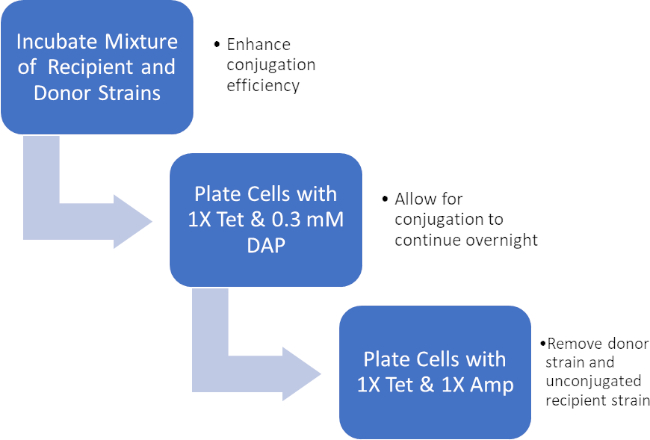
Figura 2: Esquema de protocolo. Este esquema mostra uma visão geral do protocolo apresentado.
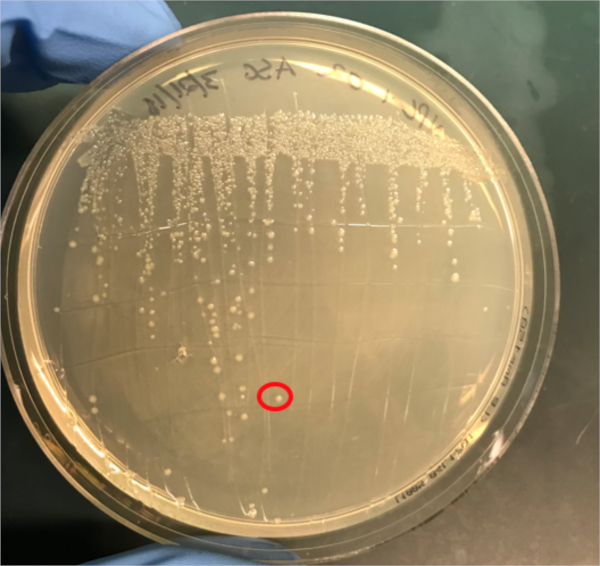
Figura 3A: A confirmação da conjugação bem sucedida pela PCR. A) Os estoques congeladores das amostras de controle conjugadas e negativas foram listrados em placas de ágar e uma colônia foi selecionada (vermelha) para isolamento de DNA.
Procedimento
1. Configuração
- Autoclave aproximadamente 1L de médium Luria-Bertani (LB). Este LB estéril será usado para fazer aproximadamente 5 mL de LB contendo ácido diaminopimelic de 0,3 mM (DAP).
- Recolher as seguintes placas: placas de ágar LB com 1X Tet e 0,3 mM DAP, placas de ágar LB apenas com 1X Tet e placas de ágar LB apenas com 1X Amp/Tet.
- Certifique-se de que um pouco de glicerol e uma caixa de pontas de pipeta de plástico pré-esterilizadas estejam próximas.
- Antes de
Resultados
Se a conjugação for bem sucedida, um produto PCR de banda de 500 pares base será observado no poço em que a reação pcr 1 foi carregada (Bem #2 na Figura 3B), enquanto nenhuma banda será observada no poço em que a reação pcr 3 foi carregada (Bem #4 na Figura 3B). A presença desta banda confirma a transferência bem sucedida do gene de resistência à ampicilina, conferindo assim resistência à ampicilina à cepa J53 de E. coli.
Log in or to access full content. Learn more about your institution’s access to JoVE content here
Aplicação e Resumo
A conjugação é um processo natural de transferência genética horizontal que depende do contato direto celular-célula de uma célula doadora e de uma célula receptora. Esse processo é compartilhado entre todos os tipos de bactérias e tem sido fundamental na evolução bacteriana, principalmente a resistência a antibióticos. Em laboratório, a conjugação pode ser usada como um método eficaz de transferência genética que é muito menos disruptivo quando comparado a outras técnicas. Fora do laboratório, a c...
Referências
- Lederberg J, Tatum, E.L. Gene recombination in Escherichia coli Nature. 1946;158:558.
- Holmes R.K. J, M.G. Genetics: Exchange of Genetic Information. 4th Edition ed. Baron S, editor. Galveston, TX: University of Texas Medical Branch at Galveston; 1996.
- Cruz F, Davies, J. Horizontal gene transfer and the origin of species: lessons from bacteria. Trends in Microbiology. 2000;8:128-33.
- Llosa M, Cruz, F. Bacterial conjugation: a potential tool for genomic engineering. Ressearch in Microbiology. 2005;156:1-6.
- Lacroix B, Citovsky, V. Transfer of DNA from Bacteria to Eukaryotes. mBio. 2016;7(4):1-9.
- Llosa M, et al. Bacterial conjugation: a two-step mechanism for
- DNA transport. Molecular Microbiology. 2002;45:1-8.
- Grohmann E, Muth, G., Espinosa, M. Conjugative Plasmid Transfer in Gram-Positive Bacteria. Microbiology and Molecular Biology Reviews. 2003;67:277-301.
- Firth N, Ippen-Ihler, K, Skurray, RA. Structure and function of the F factor and mechanism of conjugation. Escherichia coli and salmonella: cellular and molecular biology. 1996;2:2377-401.
- Smillie C, Garcillan-Barcia MP, Francia MV, Rocha EPC, De La Cruz F. Mobility of Plasmids. Microbiology and Molecular Biology Reviews. 2010;74(3):434-52.
- Cascales E. Definition of a Bacterial Type IV Secretion Pathway for a DNA Substrate. 2004;304(5674):1170-3.
- Wang P, Yu Z, Li B, Cai X, Zeng Z, Chen X, et al. Development of an efficient conjugation-based genetic manipulation system for Pseudoalteromonas. Microbial Cell Factories. 2015;14(1):11.
- Yi H, Cho YJ, Yong D, Chun J. Genome Sequence of Escherichia coli J53, a Reference Strain for Genetic Studies. Journal of Bacteriology. 2012;194(14):3742-3.
- Baumann RLB, E. H.; Wiseman, J. S.; Vaal, M.; Nichols, J. S. Inhibition of Escherichia coli Growth and Diaminopimelic Acid Epimerase by 3-Chlorodiaminopimelic Acid. Antimicrobial Agents and Chemotherapy 1988;32:1119-23.
- Rocha D, Santos, CS, Pacheco LG. Bacterial reference genes for gene expression studies by RT-qPCR: survey and analysis. Antonie Van Leeuwenhoek. 2015;108:685-93.
Pular para...
Vídeos desta coleção:

Now Playing
Conjugação: um método para transferir a resistência à ampicilina da E. coli doadora para a receptora
Microbiology
38.2K Visualizações

Criando uma coluna de Winogradsky: um método para enriquecer as espécies microbianas em uma amostra de sedimento
Microbiology
129.3K Visualizações

Diluições em série e plaqueamento: enumeração microbiana
Microbiology
315.9K Visualizações

Culturas de enriquecimento: cultivo de micróbios aeróbicos e anaeróbicos em meios seletivos e diferenciais
Microbiology
132.0K Visualizações

Culturas puras e semeadura por esgotamento: isolamento de colônias bacterianas únicas de uma amostra mista
Microbiology
166.2K Visualizações

Sequenciamento de rRNA 16S: uma técnica baseada em PCR para identificar espécies bacterianas
Microbiology
188.9K Visualizações

Curvas de crescimento: gerando curvas de crescimento usando unidades formadoras de colônias e medições de densidade óptica
Microbiology
295.7K Visualizações

Teste de sucetibilidade a antibióticos: testes de epsilômetro para determinar valores de MIC de dois antibióticos e avaliar a sinergia de antibióticos
Microbiology
93.7K Visualizações

Microscopia e Coloração: Coloração de Gram, Cápsula e Endósporo
Microbiology
363.2K Visualizações

Ensaio de placa: um método para determinar o título viral como unidades formadoras de placa (PFU)
Microbiology
186.1K Visualizações

Transformação de células de E. coli usando um protocolo adaptado de cloreto de cálcio
Microbiology
86.8K Visualizações

Transdução fágica: um método para transferir a resistência à ampicilina da E. coli doadora para a receptora
Microbiology
29.1K Visualizações
Copyright © 2025 MyJoVE Corporation. Todos os direitos reservados