Method Article
Identification of Pre-weanling 7-Day-Old Mice with Pinna Edge Biopsies
In This Article
Summary
The protocol presents a novel stepwise approach to producing a pinna-edge biopsy (PEB) in post-natal day (PND) 7 mice for the purpose of identification and simultaneous genotyping.
Abstract
Identification and genotyping of mice is often an essential part of many in vivo scientific studies. Several methods have been described for identification and genotyping in weaned mice; however, to date, there are far fewer techniques described for pre-weanling mice. Pinna Edge Biopsy (PEB) is a method of identification with subsequent use of tissue for genotyping. This article describes and provides video instruction on a stepwise approach to PEB on post-natal day (PND) 7 mice. Eight different patterns of pinna biopsy location and technique are outlined which have been shown to sustain into adulthood for identification. This article also discusses ways to optimize the PEB and shows the benign effect this technique has on the growth and development of the pups, with all mice showing appropriate weight gain without any morbidity or mortality throughout the study. This technique will provide investigators with a method for mouse identification at an age where there are few identification and genotyping methods available.
Introduction
In certain scientific studies, the method to identify and simultaneously genotype a mouse can be crucial. When using a mouse model, there are several commonly used methods that have been developed to genotype and identify animals at the same time (e.g., ear punching, ear tagging), and other methods that only identify animals (e.g., tattooing, subcutaneous transponder implantation, marker or dye application) or only genotype animals (e.g., tail biopsy)1,2. Most of these techniques are intended to be performed at the time of weaning. Techniques to simultaneously identify and genotype in younger, pre-weanling animals are fewer1,2,3,4.
To assist work that requires identification and genotyping of mice at younger ages, a study was performed to validate PEB as a reliable identification and genotyping method for PND 7 pups and compare it to the use of PEB in mice weaned at PND 215. As this study details, horizontal and vertical pinna slices were selected because a prior study using PND 7 pup carcasses found pinna patterns created using PEB were clear, quick, provided a variety of pattern combinations, identifiable, and the tissue was easy to remove5. Other methods, such as ear punches and ear notches, were also explored, but there was not enough surface area on PND 7 pups for the creation of complete circular punches or notches. This preliminary work led the referenced study to test these patterns in live PND 7 mice5. This study showed that the tissue size retrieved from the biopsy of the ear pinna can be used for successful genotyping. The PEB patterns were also intact as mice aged to PND 63, regardless of when the pinna was biopsied at PND 7 or PND 215.
The goal for the development of this technique is to provide a simultaneous identification and genotyping method for animals aged PND 7 to 14 that, according to current literature, do not have a method available. The current article, accompanied by the guided video, illustrates how to perform an ear biopsy at the pinna edge of a 7-day mouse's ear to simultaneously identify and retrieve a sufficient quantity of tissue for PCR genotyping. There are eight different biopsy combinations and one with no biopsy for a total of nine pinna patterns available for use to identify individual mice. Tips on performing pinna biopsies are further discussed to maximize success for the clear identification of the various pinna patterns in PND 7 pups.
Protocol
All procedures outlined in the protocol were performed in compliance with the NIH Guide for the Care and Use of Laboratory Animals6and approved by the Massachusetts General Hospital (MGH) Institutional Animal Care and Use Committee (IACUC). The identification method described below has been tested in C57BL/6NCrl mice of both sexes5. This method has not been used in other strains, stocks, or transgenics. Pinna biopsy identification may not be suitable for mice that have an increased healing capacity (e.g., MRL mouse strain)7.
1. Mouse pup restraint
- Ensure the mouse is PND 7 or older. If the mouse is younger than PND 7, this PEB method may not be feasible.
- Gently restrain the mouse in a manner that allows for visualization and access to the ears. Pick the pup up by the base of the tail with the user's non-dominate hand and transfer it to a stable surface.
- Apply gentle pressure along the pup's back with the thumb and forefinger of the dominant hand until the loose skin between the ears can be pinched. Pinch the skin tightly enough so that the head does not move freely, but ensure the animal is breathing normally (Figure 1).
- If the animal is being restrained with the left hand, ensure the pup is in a position that makes the right ear pinna accessible (i.e., the pup's nose is facing away from the hander's body). To access the left ear pinna, supinate the hand grasping so that the left side of the animal faces the handler.
- If the animal is being restrained with the right hand, ensure the pup is in a position that makes the left ear pinna readily accessible (i.e., the pup's nose is facing away from the handler's body). To access the right ear pinna, supinate the hand grasping the animal so that the right side of the animal faces the handler.
2. Selection of pinna biopsy pattern
- Use a total of nine patterns with eight PEB patterns and one no-biopsy pattern (Figure 2). Select and record a PEB pattern.
3. Pinna edge biopsy
- Use clean instruments for each biopsy. Small dissection scissors or McPherson-Vannas scissors are recommended (see Table of Materials). Spray the instruments with disinfectants and wipe clean with 70% isopropyl alcohol after each use.
- Hold the scissors with the dominant hand.
- To create a vertical PEB, trim the lateral edge of the pinna in a vertical direction so that approximately 1/4th to 1/3rd of the total pinnae (i.e., about 0.8 mm x 3 mm tissue) is removed from the side of the ear (Figure 3G).
- To create a horizontal PEB, trim the dorsal edge of the pinna so that approximately 1/4th to 1/3rd of the total pinna (i.e., about 0.8 mm x 3 mm tissue) is removed (Figure 3H). Make the cut at a 45° angle instead of a straight line (Figure 4). The angle accounts for ear development and growth over time and assures the pattern remains horizontal in nature.
- The eight pinna patterns are a combination of the vertical PEB and horizontal PEB on either the left, right, or both pinnae. Make the following eight pinna biopsy patterns: left vertical (LV), right vertical (RV), left horizontal (LH), right horizontal (RH), left vertical and right horizontal (LVRH), left and right vertical (LVRV), left and right horizontal (LHRH), and left horizontal and right vertical (LHRV).
- Use fine forceps to carefully deposit the excised pinna tissue into a sample container to be used for genotyping.
- If in the unlikely event that blood pools from the pinna biopsy site, with the dominant hand and while the animal is still restrained, use a piece of clean gauze or a cotton tip applicator to place pressure on the site of bleeding until the bleeding stops.
- Place the pup gently back in the cage with the dam. Observe pups for 1-2 min after placement back into the cage to assess for any further bleeding from the PEB site.
Results
Figure 1 illustrates a manual method of gently restraining PND 7 mice so their ears are visible for manipulation. The restraint uses the thumb and index finger to grasp the excess skin of the scruff. The amount of skin grasped depends on the animal's age, with less tissue available in smaller animals.
The PEB patterns listed in Figure 2 have been tested to be identifiable up to PND 63 and anecdotally have been seen to last for longer (i.e., 2 years). Figure 3 shows three mice photographed initially at PND 7 and followed till PND 63. One pup did not have a PEB, the second pup received a left-vertical (LV) PEB, and the third pup had a right-horizontal (RH) PEB. The figure shows that these PEBs are intact even until PND 63.
For horizontal PEBs, the pattern can be made more obvious by cutting the dorsal edge of the pinna at a 45° angle, as shown in Figure 4. As the animal and thus the ear grows, the pinna edge will correct the angle and appear more noticeably horizontal.
In the original study that assessed the PEBs, various clinical and behavioral parameters were observed. Figure 5 shows the weight at various ages, differentiated by sex (Figure 5A shows female mice; Figure 5B shows male mice) of PND 7 pups that received a PEB of each combination5. Mice, including the pups that did not receive a PEB, showed a significant difference in weight as they aged.

Figure 1: Restraining a PND 7 pup for PEB positioning. Representative image of gently scuffing a PND 7 mouse to ensure that ears are easily accessible for the PEBs. Please click here to view a larger version of this figure.
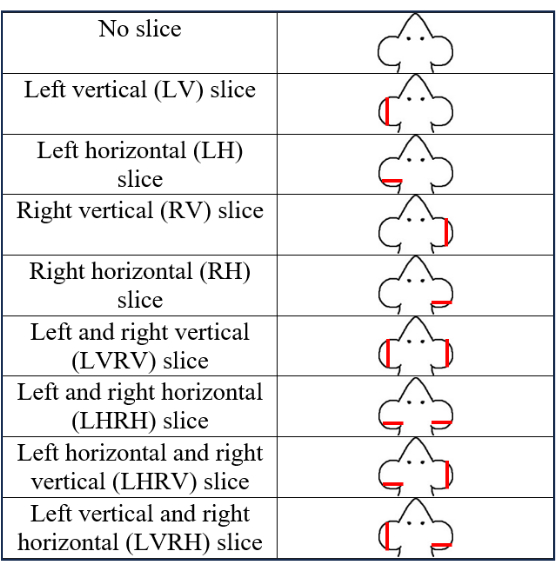
Figure 2: Illustration showing the nine pinna identification patterns on PND 7 mice. The red lines on the ears indicate the approximate locations of the biopsy. This figure has been modified from5. Please click here to view a larger version of this figure.

Figure 3: Representative images of PND 7 pups with various pinna patterns at various time points. (A, B) A PND 7 pup without a biopsy; (C, D) a PND 7 pup without a biopsy on PND 21; (E, F) a PND 7 pup without a biopsy on PND 63. (G, I, K) White arrows show a PND 7 pup with a vertical PEB on the left ear pup on PND 7, 21, and 63, respectively. (H, J, L) Red arrows show a PND 7 RH-PEB pup on PND 7, 21, and 63, respectively. This figure has been modified from5. Please click here to view a larger version of this figure.
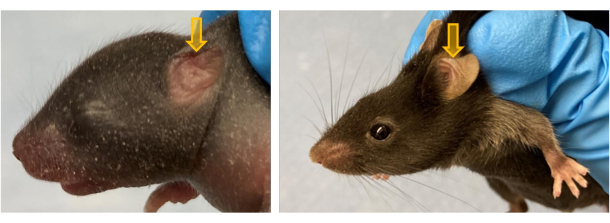
Figure 4: Horizontal PEB pattern biopsied at an angle. (A) PND 7 pup left pinna horizontal PEB is created at a 45° angle. (B) The same animal is depicted, but now it is PND 63. Note the angle has now been corrected and the PEB appears more horizontal. Please click here to view a larger version of this figure.
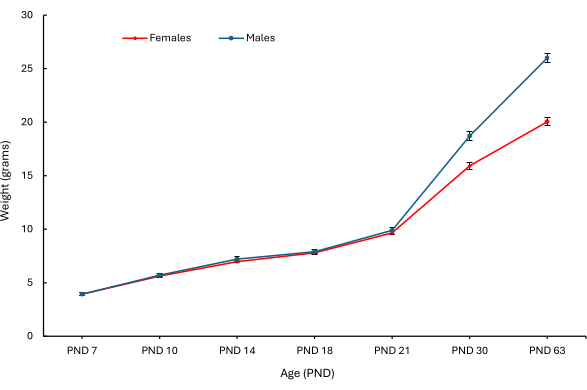
Figure 5: Mean body weights of female and male pups at PND 7, 10, 14, 18, 21, 30 and 63. The mean body weights (g) of female (red) and male (blue) mice in PND 7 pups from PND 7 to 63 were plotted by sex. Means were calculated across individual mice (n = 28 female pups; n = 26 male pups). Error bars represent SEM. This figure has been modified from5. Please click here to view a larger version of this figure.
Discussion
Significance with respect to existing methods
Previous articles have stated that for PND 14 animals and younger, their pinna may not be suitable for manipulation because of the small size2,3. While existing ear identification systems such as ear punch biopsies, ear notching, or ear tags can perform both identification and genotyping, they may require larger pinnae and, thus, older mice for identification. This novel method of PEB offers an identification method for animals at ages that previously did not have identification and genotyping methods available. No methods have been described previously in the literature for animals PND 7 to 14. Thus, PEBs offer identification as young as PND 7, and the tissue collected can be used for genotyping.
Other methods may allow for identification (e.g., temporary tail marking, tattooing, and transponder implantation) or genotyping (e.g., tail biopsy). This method allows for both identification and genotyping in just one restraining event, which further reduces stress on the animal.
Toe clipping is an option for identification and genotyping of young animals3,8,9,10. However, it should only be used when no other individual identification method is feasible, as described in the Guide for the Care and Use of Laboratory Animals6. This criterion limits the use of toe clipping. Based on this recommendation, the PEB method is an alternative to toe clipping that can be used in mice PND 7 or older.
Based on results from a previous study that is encapsulated in Figure 5, the PEB procedure did not affect the weights of the mice measured at various time points when compared to the no PEB mouse. Additionally, no morbidity or mortality was observed in the PND 7 pups at any time point. All pups that were biopsied on PND 7 had milk spots on PND 10, and dam rejection was not observed. Based on these parameters, this procedure appears to have a benign effect on the growth and development of mice when performed appropriately.
Limitations of the technique
The PEB technique only allows for eight identification combinations that also enable genotyping. The ninth pattern, the no biopsy pattern, does not allow genotyping as no tissue is procured. Therefore, other methods of identification may need to be used to supplement the additional nine combinations offered. Additional PEBs can be added. For example, having a dorsal and a horizontal biopsy on one pinna can add six extra combinations. This would provide a total of 15 combinations. However, those patterns have not been performed nor tested.
The PEBs were visible and individually distinguishable up to PND 63. While not formally monitored after PND 63, the mice were kept in the colony for up to 2 years, and anecdotally, the PEB patterns were still intact.
PEB patterns were only tested in C57BL/6NCrl mice. Like with other ear identification techniques, mice with increased wound healing capability would not be suitable for this technique7.
Critical steps within the protocol
Ensuring a firm but gentle restraint that allows for visualization of the pina is essential for proper PEB execution. PND 7 animals were noted to struggle less during restraint and during the biopsy compared to PND 21 animals5. For the horizontal combinations, it is critical to perform the slice at the 45° angle, as depicted in Figure 4, to ensure the horizontal PEBs appear horizontal as the mouse ages.
Modifications and troubleshooting
In the original study that tested the efficacy of PEBs, it was discovered that generally, at least 0.8 mm x 3 mm of the pinna was biopsied in PND 7 mice5. To ensure the identification of the PEB pattern as the mouse ages, it is recommended to take the maximal amount of tissue from the pinna as feasible.
Future applications
The PEB technique was only tested in C57BL/6Crl mice, but this application could also be used in other mouse strains or stocks if tested. As mentioned previously, further PEB patterns can be identified to add more identification patterns for dams with larger litters. Finally, this method was tested and performed by an experienced mouse handler who has performed other identification techniques (e.g., ear punch). Further studies could be developed to test the ease of this method for those who are less experienced in mouse handling.
Disclosures
The authors do not have anything to disclose.
Acknowledgements
The authors would like to thank Drs. Lori S Palley and Donna M Jarrell, the authors of the original paper in this work, for their contributions.
Materials
| Name | Company | Catalog Number | Comments |
| Dissection scissors | Kent Scientific | INS600393-G | 10 cm long |
| McPherson-Vannas Scissors | Kent Scientific | INS600124 | 8 cm long |
References
- Bonaparte, D., et al. FELASA guidelines for the refinement of methods for genotyping genetically-modified rodents: a report of the Federation of European Laboratory Animal Science Associations Working Group. Lab Anim. 47 (3), 134-145 (2013).
- Dahlborn, K., et al. Report of the federation of European laboratory animal science associations working group on animal identification. Lab Anim. 47 (1), 2-11 (2013).
- Castelhano-Carlos, M. J., Sousa, N., Ohl, F., Baumans, V. Identification methods in newborn C57BL/6 mice: a developmental and behavioural evaluation. Lab Anim. 44 (2), 88-103 (2010).
- Chen, M., Kan, L., Ledford, B. T., He, J. Q. Tattooing various combinations of ears, tail, and toes to identify mice reliably and permanently. J Am Assoc Lab Anim Sci. 55 (2), 189-198 (2016).
- Chen, D. D., Molk, D. M., Palley, L. S., Jarrell, D. M. Pinna Edge Biopsy of 7 and 21 Day Old C57BL/6 Mice as a Method for Identification and Genotyping. J Am Assoc Lab Anim Sci. 62 (5), 438-448 (2023).
- Institute for the Laboratory Animal Research. Guide for the care and use of laboratory animals. , 8th ed, National Academies Press. Washington, DC. (2011).
- Rajnoch, C., et al. Regeneration of the ear after wounding in different mouse strains is dependent on the severity of wound trauma. Dev Dyn. 226 (2), 388-397 (2003).
- Han, M., Yang, X., Lee, J., Allan, C. H., Muneoka, K. Development and regeneration of the neonatal digit tip in mice. Dev Biol. 315 (1), 125-135 (2008).
- Schaefer, D. C., Asner, I. N., Seifert, B., Bürki, K., Cinelli, P. Analysis of physiological and behavioural parameters in mice after toe clipping as newborns. Lab Anim. 44 (1), 7-13 (2010).
- Frezel, N., et al. Does toe clipping for genotyping interfere with later-in-life nociception in mice. Pain Rep. 4 (3), e740(2019).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionThis article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved