A subscription to JoVE is required to view this content. Sign in or start your free trial.
Method Article
Primary Microglia Isolation from Mixed Glial Cell Cultures of Neonatal Rat Brain Tissue
In This Article
Summary
Isolating primary microglia from the cellular heterogeneity of the brain is essential to investigate their role in both physiological and pathological conditions. This protocol describes a mechanical isolation and mixed cell culture technique that provides high yield and high purity, viable primary microglial cells for in vitro study and downstream applications.
Abstract
Microglia account for approximately 12% of the total cellular population in the mammalian brain. While neurons and astrocytes are considered the major cell types of the nervous system, microglia play a significant role in normal brain physiology by monitoring tissue for debris and pathogens and maintaining homeostasis in the parenchyma via phagocytic activity 1,2. Microglia are activated during a number of injury and disease conditions, including neurodegenerative disease, traumatic brain injury, and nervous system infection 3. Under these activating conditions, microglia increase their phagocytic activity, undergo morpohological and proliferative change, and actively secrete reactive oxygen and nitrogen species, pro-inflammatory chemokines and cytokines, often activating a paracrine or autocrine loop 4-6. As these microglial responses contribute to disease pathogenesis in neurological conditions, research focused on microglia is warranted.
Due to the cellular heterogeneity of the brain, it is technically difficult to obtain sufficient microglial sample material with high purity during in vivo experiments. Current research on the neuroprotective and neurotoxic functions of microglia require a routine technical method to consistently generate pure and healthy microglia with sufficient yield for study. We present, in text and video, a protocol to isolate pure primary microglia from mixed glia cultures for a variety of downstream applications. Briefly, this technique utilizes dissociated brain tissue from neonatal rat pups to produce mixed glial cell cultures. After the mixed glial cultures reach confluency, primary microglia are mechanically isolated from the culture by a brief duration of shaking. The microglia are then plated at high purity for experimental study.
The principle and protocol of this methodology have been described in the literature 7,8. Additionally, alternate methodologies to isolate primary microglia are well described 9-12. Homogenized brain tissue may be separated by density gradient centrifugation to yield primary microglia 12. However, the centrifugation is of moderate length (45 min) and may cause cellular damage and activation, as well as, cause enriched microglia and other cellular populations. Another protocol has been utilized to isolate primary microglia in a variety of organisms by prolonged (16 hr) shaking while in culture 9-11. After shaking, the media supernatant is centrifuged to isolate microglia. This longer two-step isolation method may also perturb microglial function and activation. We chiefly utilize the following microglia isolation protocol in our laboratory for a number of reasons: (1) primary microglia simulate in vivo biology more faithfully than immortalized rodent microglia cell lines, (2) nominal mechanical disruption minimizes potential cellular dysfunction or activation, and (3) sufficient yield can be obtained without passage of the mixed glial cell cultures.
It is important to note that this protocol uses brain tissue from neonatal rat pups to isolate microglia and that using older rats to isolate microglia can significantly impact the yield, activation status, and functional properties of isolated microglia. There is evidence that aging is linked with microglia dysfunction, increased neuroinflammation and neurodegenerative pathologies, so previous studies have used ex vivo adult microglia to better understand the role of microglia in neurodegenerative diseases where aging is important parameter. However, ex vivo microglia cannot be kept in culture for prolonged periods of time. Therefore, while this protocol extends the life of primary microglia in culture, it should be noted that the microglia behave differently from adult microglia and in vitro studies should be carefully considered when translated to an in vivo setting.
Protocol
1. Dissection of Neonatal Rat Brain Tissue
- Chill Leibovitz's L-15 conditioned media (Leibowitz L-15 + 0.1% BSA + 1% Pen/Strep) to 4 °C. Warm culture media (DMEM + 10% FBS + 1% Penicillin/Streptomycin) to 37 °C. Prepare 4 60x15 mm Petri dishes with 4-5 ml of the conditioned L-15 media on ice. Sterilize all surgical tools with 100% ethanol.
- Rinse the P2 neonatal rap pups with 70% ethanol. (Neonatal rat pups aged between P1-P5 can be used in this protocol.)
- Quickly decapitate a rat pup with sterile sharp scissors and drop the head immediately into 70% ethanol. Repeat for a total of 5 rat pups then transfer heads into saline solution.
- Remove the whole brains from the heads and place into a Petri dish with 4 - 5 ml L-15 solution (Leibowitz L-15 + 0.1% BSA + 1% Pen/Strep) on ice.
- Repeat procedures 1.2 - 1.4 for the remaining pups in the litter.
- Remove the meninges from the brain and transfer the desired brain tissue (i.e., cortex, cerebellum, whole brain, etc.) into a new Petri dish with 4-5 ml of L-15 solution (Leibowitz L-15 + 0.1% BSA + 1% Pen/Strep) on ice.
2. Preparation of Mixed Glial Cell Population
- Without damaging brain tissue with scissors or a blade, transfer the tissue with a 10 ml pipette to a sterile 50 ml conical tube.
- Centrifuge conical at 2,500 RCF for 5 min at 4 °C.
- Aspirate supernatant. Add 4-5 ml of fresh L-15 media (Leibowitz L-15 + 0.1% BSA + 1% Pen/Strep).
- Pipette tissue up and down 10 times with a sterile 10 ml pipette.
- Place a cell strainer (100 μm pores) onto a fresh 50 ml conical tube. Pipette tissue up and down once with a sterile 5 ml pipette and with the pipette flush to the cell strainer, dispense the material through the cell strainer into the conical tube.
- Rinse the cell strainer with 4-5 ml of fresh L-15 media (Leibowitz L-15 + 0.1% BSA + 1% Pen/Strep).
- Centrifuge conical with strained cells at 2,500 RCF for 5 min at 4 °C.
3. Plating and Maintenance of Mixed Glial Cell Cultures
- For each rat pup brain processed, prepare 1 sterile T-75 flask by adding 12 ml of culture media (DMEM + 10% FBS + 1% Penicillin/Streptomycin) into each flask.
- Aspirate the supernatant from the pelleted cells and add 5-6 ml of culture media to the cell pellet. Pipette up and down 10 times with a 10 ml pipette.
- Transfer an equal volume of cell suspension to each T-75 flask.
- Incubate flasks in a 5% CO2 incubator at 37 °C for a total of 1-3 weeks.
- Flasks should be first incubated for 5 days, and on the fifth day, replace the culture media in each flask with 12 ml of fresh media and return to the incubator. Then, every 3 days, replace the conditioned media in each flask with 12 ml of fresh media to achieve confluence. This must be done very carefully without touching the bottom of the flasks where the cells attach.
4. Isolation and Plating of Primary Microglia
- After mixed glial cultures are completely confluent, remove flasks from the incubator and cover flask caps with parafilm to prevent gas exchange with environmental air.
- Shake flasks at 100 rps (Lab Companion SI-600, Jeio Tech) for 1 hr at 37 °C.
- Collect media from all flasks with a 10 ml pipette without disrupting the astrocyte layer on the flask surface. Place the media in 50 ml conical tubes. Add fresh media to the flasks and return flasks to the incubator.
- Centrifuge conical tubes at 2,500 RCF for 5 min at 4 °C.
- Aspirate supernatant from all conical tubes. Cell pellets are high purity microglia cells. Resuspend pellets in 1 ml of microglia plating media (DMEM + 10% FES + 1% Penicillin/Streptomycin).
- Count the cell density in the resuspended media using a hemocytometer.
- Add an appropriate volume of microglia plating media to achieve a 2 x 10e5 cells per ml density. Plate as appropriate for experimental analysis.
- Allow microglia to attach overnight.
5. Representative Results
The protocol described above results in high purity primary microglia cultures. As determined by immunohistochemistry for a macrophage/microglia cell specific marker (Iba1), plated microglia cultures are > 90% pure (Figure 2). Additionally, staining for astrocyte, oligodendrocyte and neuron cell specific markers demonstrates minimal contamination (Figure 2). After establishing mixed glial cell cultures, microglia can be isolated by shaking up to 4 successive times. The initial microglia isolation will yield approximately 2.2 x 10ˆ6 cells/ml or approximately 9 million cells per 10 flasks and the expected yield decreases with each successive shake. Microglia isolated by this method have been used successfully to investigate phagocytosis ability, function such as nitric oxide production, and neuronal toxicity (Figure 3) 13-14.
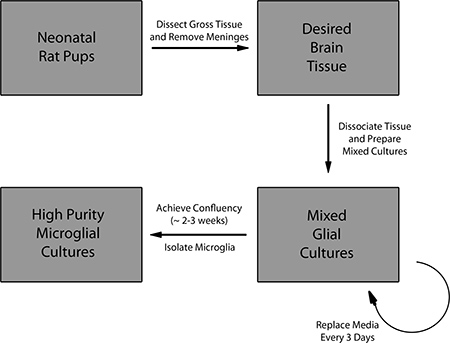
Figure 1. Overview of the protocol for preparing mixed glial cell cultures and isolation of primary microglia.
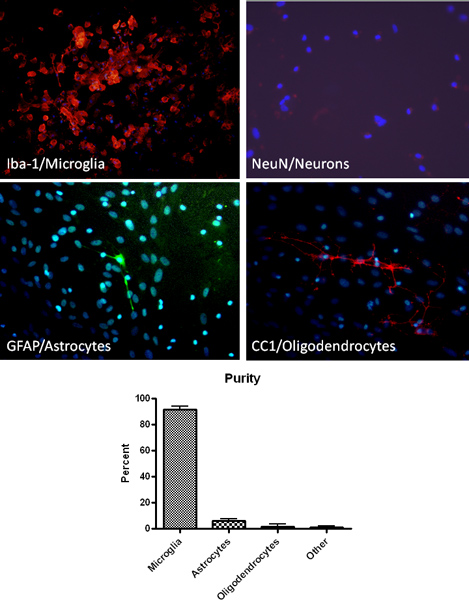
Figure 2. Isolation of high purity microglia as verified by immunohistochemical analysis using microglia (Iba-1, red), astrocyte (GFAP, green), neuron (NeuN, red) and oligodendrocyte (CC1, red) specific markers. Staining for DNA of cells in culture by DAPI (blue) is also shown.
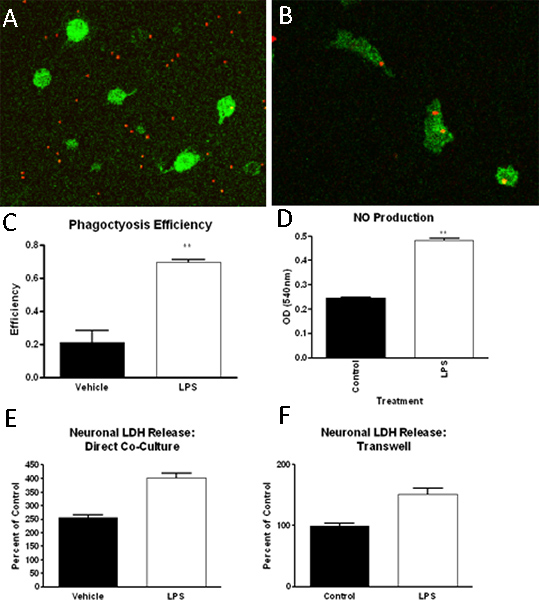
Figure 3. Microglia isolated in the method described have been used in a number of assays, including, but not limited to, phagocytosis assays, function assays and neurotoxicity studies. In these studies, we have found that microglia (Iba-1 positive, green), when exposed to control (A) or LPS (B) treated media, increase their phagocytosis of fluorescently labeled latex beads (red), which can be quantified (C). Further, LPS (1ng/ml) treatment can increase nitric oxide production in microglia (D). Finally, via both transwell insert-separated or direct microglia-neuron cultures, we have found that microglia incubated with LPS can induce neuronal cell death as measured by lactate dehydrogenase (LDH) release (E, F).
Discussion
While this protocol is routinely utilized to produce pure and healthy microglia for research experiments, careful consideration of technical aspects during critical parts of the procedure will minimize variability in the isolated microglia. First, during the dissection of brain tissue from neonatal rat pups, working in a timely fashion is necessary to minimize hypoxic and ischemic damage to the tissue. However, it is also important to completely remove the meningeal covering from the brain during dissection, beca...
Disclosures
No conflicts of interest declared.
Acknowledgements
Funded by the intramural program at the Uniformed Services University.
Materials
| Name | Company | Catalog Number | Comments |
| 60 mm x 15 mm Petri dishes | Fisher brand | 0875713A | |
| Sharp dissecting scissors | Fine Science Tools | 14094-11 | |
| Dumont #7b forceps- standard tips, curved, 11cm | Fine Science Tools | 11270-20 | |
| Dumont #5 forceps-standard tips, straight, 11 cm | Fine Science Tools | 11251-10 | |
| 50 ml conical centrifuge tubes | VWR | 89039-656 | |
| 5 ml serological pipettes | Grenier Bio One | 606180 | |
| 10 ml serological pipettes | Grenier Bio One | 607180 | |
| 100 μm sterile nylon cell strainer | Falcon | 35-2360 | |
| 75 cm2 tissue culture flasks | Corning | 430641 | |
| Dulbecco's minimal essential medium (DMEM) | Gibco (Invitrogen) | 31053-028 | |
| Leibovitz's L-15 medium | Gibco (Invitrogen) | 11415064 | |
| Fetal bovine serum | Gibco(Invitrogen) | 16000-036 | |
| Fetal equine serum | Fisher | SH3007402 | |
| Penicillin-Streptomycin | Gibco (Invitrogen) | 15140163 | |
| 100% Ethanol | The Warner Graham Company | 64-17-5 | |
| Phosphate buffered saline solution, 10X, pH 7.4 | Quality Biological, inc. | 119-069-131 | 1X in sterile, distilled water |
| Biohazard bags | VWR | 14220-028 | |
| Haemocytometer | Hausser Scientific | 1492 | |
| 6-well cell culture plates with cellBIND surface | Corning | 3335 |
References
- Ransohoff, R. M., Perry, V. H. Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 27, 119-145 (2009).
- Raivich, G., Bohatschek, M., Kloss, C. U., Werner, A., Jones, L. L., Kreutzberg, G. W. Neuroglial activation repertoire in the injured brain: graded response, molecular mechanisms and cues to physiological function. Brain Res. Brain Res. Rev. 30, 77-105 (1999).
- Loane, D. J., Byrnes, K. R. Role of microglia in neurotrauma. Neurotherapeutics. 7, 245-265 (2010).
- Glass, C. K., Saijo, K., Winner, B., Marchetto, M. C., Gage, F. H. Mechanisms underlying inflammation in neurodegeneration. Cell. 140, 918-934 (2010).
- Pocock, J. M., Liddle, A. C. Microglial signalling cascades in neurodegenerative disease. Prog. Brain Res. 132, 555-565 (2001).
- Block, M. L., Hong, J. S. Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem. Soc. Trans. 35, 1127-1132 (2007).
- Barger, S. W., Basile, A. S. Activation of microglia by secreted amyloid percursor protein evokes release of glutamate by cystine exchange and attenuates synpatic function. J. Neurochem. 76, 846-854 (2001).
- Ni, M., Aschner, M. Neonatal rat primary microglia: isolation, culturing, and selected applications. Curr. Protoc. Toxicol. Chapter 12, Unit 12.17 (2010).
- Hassan, N. F., Rifat, S., Campbell, D. E., McCawley, L. J., Douglas, S. D. Isolation and flow cytometric characterization of newborn mouse brain-derived microglia maintained in vitro. J. Leukoc. Biol. 50, 86-92 (1991).
- Hassan, N. F., Prakash, K., Chehimi, J., McCawley, L. J., Douglas, S. D. Isolation and characterization of newborn rabbit brain-derived microglia. Clin. Immunol. Immunopathol. 59, 426-435 (1991).
- Hassan, N. F., Campbell, D. E., Rifat, S., Douglas, S. D. Isolation and characterization of human fetal brain-derived microglia in in vitro culture. Neuroscience. 41, 149-158 (1991).
- Frank, M. G., Wieseler-Frank, J. L., Watkins, L. R., Maier, S. F. Rapid isolation of highly enriched and quiescent microglia from adult rat hippocampus: immunophenotypic and functional characteristics. J. Neurosci. Methods. 151, 121-130 (2006).
- Byrnes, K. R., Stoica, B., Loane, D. J., Riccio, A., Davis, M. I., Faden, A. I. Metabotropic glutamate receptor 5 activation inhibits microglial associated inflammation and neurotoxicity. Glia. 57, 550-560 (2009).
- Loane, D. J., Stoica, B. A., Pajoohesh-Ganji, A., Byrnes, K. R., Faden, A. I. Activation of metabotropic glutamate receptor 5 modulates microglial reactivity and neurotoxicity by inhibiting NADPH oxidase. J. Biol. Chem. 284, 15629-15639 (2009).
Reprints and Permissions
Request permission to reuse the text or figures of this JoVE article
Request PermissionExplore More Articles
This article has been published
Video Coming Soon
Copyright © 2025 MyJoVE Corporation. All rights reserved